Vemurafenib
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛməˈræfənɪb/VEM-ə-RAF-ə-nib |
| Trade names | Zelboraf |
| Other names | PLX4032, RG7204, PLX4720, RO5185426 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.287.801 |
| Chemical and physical data | |
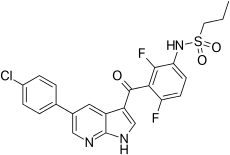
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
| vemurafenib | |
|---|---|
| Drug mechanism | |
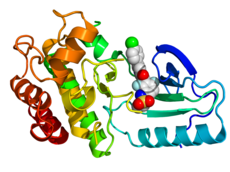 Crystallographic structureof B-Raf (rainbow colored,N-terminus= blue,C-terminus= red) complexed with vemurafenib (spheres, carbon = white, oxygen = red, nitrogen = blue, chlorine = green, fluorine = cyan, sulfur = yellow).[2] | |
| Therapeutic use | melanoma |
| Biological target | BRAF |
| Mechanism of action | protein kinase inhibitor |
| External links | |
| PDBligand id | 032:PDBe,RCSB PDB |
| LIGPLOT | 3og7 |
Vemurafenib(INN), sold under the brand nameZelboraf,is amedicationused for the treatment of late-stagemelanoma.[2]It is an inhibitor of theB-Rafenzymeand was developed byPlexxikon.[2]
Mechanism of action
[edit]Vemurafenib causesprogrammed cell deathinmelanomacell lines.[3]Vemurafenib interrupts theB-Raf/MEK stepon theB-Raf/MEK/ERK pathway− if the B-Raf has the common V600E mutation.
Vemurafenib only works in melanoma patients whose cancer has a V600E BRAF mutation (that is, atamino acidposition number 600 on the B-Raf protein, the normalvalineis replaced byglutamic acid).[4]About 60% of melanomas have this mutation. It also has efficacy against the rarer BRAF V600K mutation. Melanoma cells without these mutations are not inhibited by vemurafenib; the drug paradoxically stimulates normal BRAF and may promote tumor growth in such cases.[5][6]
Resistance
[edit]Three mechanisms of resistance to vemurafenib (covering 40% of cases) have been discovered:
- Cancer cells begin to overexpress cell surface proteinPDGFRB,creating an alternative survival pathway.
- A secondoncogenecalledNRASmutates, reactivating the normal BRAF survival pathway.[7]
- Stromal cellsecretion ofhepatocyte growth factor(HGF).[8][9]
Side effects
[edit]At the maximum tolerated dose (MTD) of 960 mg twice a day 31% of patients get skin lesions that may need surgical removal.[2]The BRIM-2 trial investigated 132 patients; the most common adverse events werearthralgiain 58% of patients, skin rash in 52%, and photosensitivity in 52%. In order to better manage side effects some form of dose modification was necessary in 45% of patients. The median daily dose was 1750 mg, 91% of the MTD.[10]
History
[edit]In aphase I clinical study,vemurafenib (then known as PLX4032) was able to reduce numbers of cancer cells in over half of a group of 16 patients with advanced melanoma. The treated group had a median increased survival time of 6 months over the control group.[11][12][13][14]
A second phase I study, in patients with a V600E mutation in B-Raf, ~80% showed partial to complete regression. The regression lasted from 2 to 18 months.[15]
In early 2010 aPhase Itrial[16]for solid tumors (includingcolorectal cancer), and aphase IIstudy (for metastatic melanoma) were ongoing.[17]
A phase III trial (vsdacarbazine) in patients with previously untreated metastatic melanoma showed an improved rates of overall and progression-free survival.[18]
In June 2011, positive results were reported from the phase III BRIM3 BRAF-mutation melanoma study.[19]The BRIM3 trial reported good updated results in 2012.[20]
Further trials are planned including a trial of vemurafenib co-administered with GDC-0973 (cobimetinib), aMEK-inhibitor.[19]After good results in 2014, the combination was submitted to the European Medical Agency and the USFood and Drug Administrationfor marketing approval.[21]
In January 2015, trial results compared vemurafenib with the combination ofdabrafenibandtrametinibfor metastatic melanoma.[22]
Society and culture
[edit]Legal status
[edit]Vemurafenib was approved in the United States for the treatment of late-stage melanoma in August 2011,[23]making it the first drug designed usingfragment-based lead discoveryto gain regulatory approval.[24]
Vemurafenib was approved for use in Canada in February 2012.[25]
In February 2012, theEuropean Commissionapproved vemurafenib as a monotherapy for the treatment of adults with BRAFV600Emutationpositive unresectable ormetastatic melanoma,the most aggressive form of skin cancer.[26]
In November 2017, the USFood and Drug Administration(FDA) approved vemurafenib for the treatment of people withErdheim–Chester disease(ECD), a rare type of histiocytic neoplasm.[27][28]
Research
[edit]A trial combining vemurafenib and ipilimumab was stopped in April 2013 because of signs ofliver toxicity.[29]
Treating Hairy Cell Leukemia
[edit]In 2012, a grant from theHairy cell leukemiaFoundation supported the discovery of the BRAF mutation in classic HCL. This discovery charted a new path forward for many patients. It improved diagnosis and opened the door for additional therapies to be used in managing HCL.[30]In a phase II clinical trial, Memorial Sloan Kettering is testing Vemurafenib, plus Obinutuzumab, in patients with previously untreated classical hairy cell leukemia.[31]A separate clinical study treatment with only Vemurafenib (or monotherarpy) demonstrated high response rates in relapsed/refractory (R/R) hairy cell leukemia (HCL), achieving an overall response rate of 86%, including 33% complete response (CR) and 53% partial response. However, after a median follow-up of 40 months, 21 of 31 responders (68%) experienced relapse with a median relapse-free survival (RFS) of 19 months (range, 12.5-53.9 months).[32]
References
[edit]- ^ab"Australian Product Information: Zelboraf® (vemurafenib)".Roche Products Pty Limited.25 March 2020.
- ^abcdPDB:3OG7;Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. (September 2010)."Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma".Nature.467(7315): 596–599.Bibcode:2010Natur.467..596B.doi:10.1038/nature09454.PMC2948082.PMID20823850.
- ^Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C (May 2008). "BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells".Molecular Cancer Research.6(5): 751–759.doi:10.1158/1541-7786.MCR-07-2001.PMID18458053.S2CID16031942.
- ^Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, et al. (May 2015)."Metastatic melanoma - a review of current and future treatment options".Acta Dermato-Venereologica.95(5): 516–524.doi:10.2340/00015555-2035.PMID25520039.
- ^Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. (March 2010)."RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth".Nature.464(7287): 431–435.Bibcode:2010Natur.464..431H.doi:10.1038/nature08833.PMID20130576.
- ^Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. (April 2010)."PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells".Pigment Cell & Melanoma Research.23(2): 190–200.doi:10.1111/j.1755-148X.2010.00685.x.PMC2848976.PMID20149136.
- ^Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. (December 2010)."Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation".Nature.468(7326): 973–977.Bibcode:2010Natur.468..973N.doi:10.1038/nature09626.PMC3143360.PMID21107323.
- "Researchers Uncover Drug-Resistance Mechanisms in BRAF Melanoma".Genetic Engineering & Biotechnology News.November 25, 2010.
- ^Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. (July 2012)."Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion".Nature.487(7408): 500–504.Bibcode:2012Natur.487..500S.doi:10.1038/nature11183.PMC3711467.PMID22763439.
- ^Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. (July 2012)."Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors".Nature.487(7408): 505–509.Bibcode:2012Natur.487..505W.doi:10.1038/nature11249.PMC3724525.PMID22763448.
- ^"BRIM-2 Upholds Benefits Emerging with Vemurafenib in Melanoma".Oncology & Biotech News.5(7). July 2011.
- ^"Drug hope for advanced melanoma".BBC News. 2009-06-02.Retrieved2009-06-07.
- ^Harmon A (2010-02-21)."A Roller Coaster Chase for a Cure".The New York Times.
- ^Garber K (December 2009). "Cancer research. Melanoma drug vindicates targeted approach".Science.326(5960): 1619.Bibcode:2009Sci...326.1619G.doi:10.1126/science.326.5960.1619.PMID20019269.
- ^Flaherty K."Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer".2009 ASCO Annual Meeting Abstract, J Clin Oncol 27:15s, 2009 (suppl; abstr 9000).Archived fromthe originalon 2013-01-27.Retrieved2010-09-10.
- ^Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. (August 2010)."Inhibition of mutated, activated BRAF in metastatic melanoma".The New England Journal of Medicine.363(9): 809–819.doi:10.1056/NEJMoa1002011.PMC3724529.PMID20818844.
- Lowe D (September 9, 2010)."PLX4032: The Good News and the Bad News".In the Pipeline.Corante.Archived fromthe originalon 2010-09-11.
- ^Clinical trial numberNCT00405587for "Safety Study of PLX4032 in Patients With Solid Tumors" atClinicalTrials.gov
- ^Clinical trial numberNCT00949702for "A Study of RO5185426 in Previously Treated Patients With Metastatic Melanoma" atClinicalTrials.gov
- ^"Plexxikon Announces First Patient Dosed in Phase 3 Trial of PLX4032 (RG7204) for Metastatic Melanoma"(Press release). Plexxikon. 2010-01-08. Archived fromthe originalon 2020-12-01.Retrieved2011-02-03.
- ^ab"Plexxikon and Roche Report Positive Data from Phase III BRAF Mutation Melanoma Study".Genetic Engineering & Biotechnology News.Mary Ann Liebert Publishers. 6 June 2011.
- ^"Vemurafenib Improves Overall Survival in Patients with Metastatic Melanoma".Archived fromthe originalon 2022-01-11.Retrieved2012-12-17.
- ^"Cobimetinib at exelixis.com".Archived fromthe originalon 2015-02-04.Retrieved2015-02-04.
- ^"MEK/BRAF Inhibitor Combo Reduces Death by One-Third in Melanoma".2015.
- ^"FDA Approves Zelboraf (Vemurafenib) and Companion Diagnostic for BRAF Mutation-Positive Metastatic Melanoma, a Deadly Form of Skin Cancer"(Press release). Genentech.Retrieved2011-08-17.
- ^Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P (November 2012). "Vemurafenib: the first drug approved for BRAF-mutant cancer".Nature Reviews. Drug Discovery.11(11): 873–886.doi:10.1038/nrd3847.PMID23060265.S2CID9337155.
- ^"Notice of Decision for ZELBORAF".Health Canada.11 March 2012. Archived fromthe originalon 2 May 2012.Retrieved21 April2012.
- ^Hofland P (February 20, 2012)."First Personalized Cancer Medicine Allows Patients with Deadly Form of Metastatic Melanoma to Live Significantly Longer".Onco'Zine.The International Cancer Network. Archived fromthe originalon April 11, 2012.RetrievedFebruary 18,2013.
- ^"FDA approves first treatment for certain patients with Erdheim–Chester disease, a rare blood cancer".U.S.Food and Drug Administration(FDA)(Press release).Retrieved2018-05-20.
- ^Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, et al. (March 2018)."Vemurafenib for BRAF V600-Mutant Erdheim-Chester Disease and Langerhans Cell Histiocytosis: Analysis of Data From the Histology-Independent, Phase 2, Open-label VE-BASKET Study".JAMA Oncology.4(3): 384–388.doi:10.1001/jamaoncol.2017.5029.PMC5844839.PMID29188284.
- ^"Getting close and personal".The Economist.January 4, 2014.ISSN0013-0613.Retrieved2016-04-15.
- ^"Hairy Cell Leukemia: Celebrating Progress?".HCLF Blog.July 29, 2022.RetrievedJuly 25,2022.
- ^Clinical trial numberNCT03410875for "Hairy Cell Leukemia with Vemurafebib" atClinicalTrials.gov
- ^Handa S, Lee JO, Derkach A, Stone RM, Saven A, Altman JK, et al. (December 2022)."Long-term outcomes in patients with relapsed or refractory hairy cell leukemia treated with vemurafenib monotherapy".Blood.140(25): 2663–2671.doi:10.1182/blood.2022016183.PMC9935554.PMID35930750.
Further reading
[edit]- Dean L (2017)."Vemurafenib Therapy and BRAF and NRAS Genotype".In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.).Medical Genetics Summaries.National Center for Biotechnology Information(NCBI).PMID28809522.Bookshelf ID: NBK447416.
