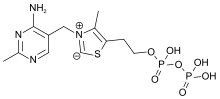
Thiamine
 | |
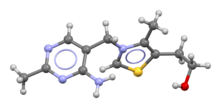 Skeletal formula and ball-and-stick model of the thiaminecation | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈθaɪ.əmɪn/THY-ə-min |
| Other names | Vitamin B1,aneurine, thiamin |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | by mouth, IV, IM[1] |
| Drug class | vitamin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Bioavailability | 3.7% to 5.3% (Thiamine hydrochloride)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard(EPA) |
|
| Chemical and physical data | |
| Formula | C12H17N4OS+ |
| Molar mass | 265.36g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
Thiamine,also known asthiaminandvitamin B1,is avitamin,anessential micronutrientfor humans and animals.[3][4]It is found in food and commercially synthesized to be adietary supplementormedication.[1][5]Phosphorylatedforms of thiamine are required for somemetabolic reactions,including the breakdown ofglucoseandamino acids.[1]
Food sources of thiamine includewhole grains,legumes,and some meats and fish.[1][6]Grain processingremoves much of the vitamin content, so in many countriescerealsandfloursareenrichedwith thiamine.[1]Supplements and medications are available to treat and preventthiamine deficiencyand the disorders that result from it such asberiberiandWernicke encephalopathy.They are also used to treatmaple syrup urine diseaseandLeigh syndrome.Supplements and medications are typically takenby mouth,but may also be given byintravenousorintramuscular injection.[7]
Thiamine supplements are generally well tolerated.Allergic reactions,includinganaphylaxis,may occur when repeated doses are given by injection.[7][8]Thiamine is on theWorld Health Organization's List of Essential Medicines.[9]It is available as ageneric medication,and in some countries as a non-prescription dietary supplement.[7]
Definition
[edit]Thiamine is one of theB vitaminsand is also known as vitamin B1.[3][4]It is acationthat is usually supplied as achloridesalt.It issolublein water,methanolandglycerol,but practically insoluble in less polarorganic solvents.[10][11]In the body, thiamine can formderivatives;the most well-characterized of which isthiamine pyrophosphate(TPP), acoenzymein thecatabolismof sugars and amino acids.[3]
The chemical structure consists of anaminopyrimidineand athiazoliumring linked by amethylene bridge.The thiazole is substituted with methyl andhydroxyethyl side chains.Thiamine is stable at acidicpH,but it is unstable inalkaline solutionsand from exposure toheat.[10][11]It reacts strongly inMaillard-type reactions.[10]Oxidationyields the fluorescent derivativethiochrome,which can be used to determine the amount of the vitamin present in biological samples.[12]
Deficiency
[edit]Well-known disorders caused by thiamine deficiency includeberiberi,Wernicke–Korsakoff syndrome,optic neuropathy,Leigh's disease,African seasonalataxia(or Nigerian seasonal ataxia), andcentral pontine myelinolysis.[13]Symptoms includemalaise,weight loss, irritability and confusion.[10][14][15]
In Western countries, chronic alcoholism is a risk factor for deficiency. Also at risk are older adults, persons withHIV/AIDSordiabetes,and those who have hadbariatric surgery.[1]Varying degrees of thiamine insufficiency have been associated with the long-term use ofdiuretics.[16][17]
Biological functions
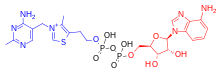
[edit]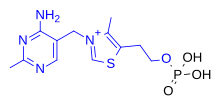
Five natural thiamine phosphate derivatives are known:thiamine monophosphate(ThMP), thiamine pyrophosphate (TPP),thiamine triphosphate(ThTP),adenosine thiamine diphosphate(AThDP) andadenosine thiamine triphosphate(AThTP). They are involved in many cellular processes.[18]The best-characterized form is TPP, acoenzymein thecatabolismof sugars and amino acids. While its role is well-known, the non-coenzyme action of thiamine and derivatives may be realized through binding to proteins which do not use that mechanism.[19]No physiological role is known for the monophosphate except as an intermediate in cellular conversion of thiamine to the di- and triphosphates.[20]
Thiamine pyrophosphate
[edit]Thiamine pyrophosphate (TPP), also called thiamine diphosphate (ThDP), participates as a coenzyme in metabolic reactions, including those in whichpolarity inversiontakes place.[21]Its synthesis is catalyzed by the enzymethiamine diphosphokinaseaccording to the reaction thiamine + ATP → TPP + AMP (EC 2.7.6.2). TPP is acoenzymefor several enzymes that catalyze the transfer of two-carbon units and in particular thedehydrogenation(decarboxylationand subsequent conjugation withcoenzyme A) of 2-oxoacids (alpha-keto acids). The mechanism of action of TPP as a coenzyme relies on its ability to form anylide.[22]Examples include:
- Present in most species
- Present in some species:
- pyruvate decarboxylase(inyeast)
- several additionalbacterialenzymes
The enzymes transketolase, pyruvate dehydrogenase (PDH), and 2-oxoglutarate dehydrogenase (OGDH) are important incarbohydrate metabolism.PDH links glycolysis to thecitric acid cycle.OGDH catalyzes the overall conversion of2-oxoglutarate(alpha-ketoglutarate) tosuccinyl-CoAand CO2during thecitric acid cycle.The reaction catalyzed by OGDH is a rate-limiting step in the citric acid cycle. The cytosolic enzyme transketolase is central to thepentose phosphate pathway,a major route for the biosynthesis of the pentosesugarsdeoxyriboseandribose.The mitochondrial PDH and OGDH are part of biochemical pathways that result in the generation ofadenosine triphosphate(ATP), which is the main energy transfer molecule for the cell. In the nervous system, PDH is also involved in the synthesis ofmyelinand the neurotransmitteracetylcholine.[11]
Thiamine triphosphate
[edit]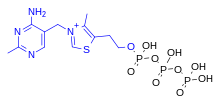
ThTP is implicated inchloride channelactivation in the neurons of mammals and other animals, although its role is not well understood.[20]ThTP has been found in bacteria, fungi and plants, suggesting that it has other cellular roles.[23]InEscherichia coli,it is implicated in the response to amino acid starvation.[24]
Adenosine derivatives
[edit]AThDP exists in small amounts in vertebrate liver, but its role remains unknown.[24]
AThTP is present inE. coli,where it accumulates as a result of carbon starvation. In this bacterium, AThTP may account for up to20% of total thiamine. It also exists in lesser amounts inyeast,roots of higher plants and animal tissue.[24]
Medical uses
[edit]During pregnancy, thiamine is sent to thefetusvia theplacenta.Pregnant women have a greater requirement for the vitamin than other adults, especially during thethird trimester.Pregnant women withhyperemesis gravidarumare at an increased risk of thiamine deficiency due to losses when vomiting.[25]Inlactatingwomen, thiamine is delivered in breast milk even if it results in thiamine deficiency in the mother.[4][26]
Thiamine is important not only formitochondrial membranedevelopment, but also forsynaptic membranefunction.[27]It has also been suggested that a deficiency hinders brain development in infants and may be a cause ofsudden infant death syndrome.[20]
Dietary recommendations
[edit]| US National Academy of Medicine | |
| Age group | RDA (mg/day) |
|---|---|
| Infants 0–6 months | 0.2* |
| Infants 6–12 months | 0.3* |
| 1–3 years | 0.5 |
| 4–8 years | 0.6 |
| 9–13 years | 0.9 |
| Females 14–18 years | 1.0 |
| Males 14+ years | 1.2 |
| Females 19+ years | 1.1 |
| Pregnant/lactating females 14–50 | 1.4 |
| * Adequate intake for infants, as an RDA has yet to be established[4] | |
| European Food Safety Authority | |
| Age group | Adequate intake (mg/MJ)[28] |
| All persons 7 months+ | 0.1 |
| Neither the US National Academy of Medicine nor the European Food Safety Authority have determined the tolerable upper intake level for thiamine[4] | |
TheUS National Academy of Medicineupdated the Estimated Average Requirements (EARs) andRecommended Dietary Allowances(RDAs) for thiamine in 1998. The EARs for thiamine for women and men aged 14 and over are 0.9 mg/day and 1.1 mg/day, respectively; the RDAs are 1.1 and 1.2 mg/day, respectively. RDAs are higher than EARs to provide adequate intake levels for individuals with higher than average requirements. The RDA during pregnancy and for lactating females is 1.4 mg/day. For infants up to the age of 12 months, the Adequate Intake (AI) is 0.2–0.3 mg/day and for children aged 1–13 years the RDA increases with age from 0.5 to 0.9 mg/day.[4]
TheEuropean Food Safety Authority(EFSA) refers to the collective set of information asDietary Reference Values,with Population Reference Intakes (PRIs) instead of RDAs, and Average Requirements instead of EARs. For women (including those pregnant or lactating), men and children the PRI is 0.1 mg thiamine per megajoule (MJ) of energy in their diet. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories ought to be consuming 1.0 mg thiamine. This is slightly lower than the US RDA.[29]
Neither the National Academy of Medicine nor EFSA have set an upper intake level for thiamine, as there is no human data for adverse effects from high doses.[4][28]
Safety
[edit]Thiamine is generally well tolerated and non-toxic whenadministered orally.[7]There are rare reports of adverse side effects when thiamine is givenintravenously,including allergic reactions,nausea,lethargy,andimpaired coordination.[28][3]
Labeling
[edit]For US food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value. Since May 27, 2016, the Daily Value has been 1.2 mg, in line with the RDA.[30][31]
Sources
[edit]Thiamine is found in a wide variety of processed and whole foods,[18]includinglentils,peas,whole grains,pork,andnuts.[6][32]A typical daily prenatal vitamin product contains around 1.5 mg of thiamine.[33]
Food fortification
[edit]Some countries require or recommend fortification of grain foods such aswheat,riceormaize(corn) because processing lowers vitamin content.[34]As of February 2022, 59 countries, mostly in North and Sub-Saharan Africa, require food fortification of wheat, rice or maize with thiamine or thiamine mononitrate. The amounts stipulated range from 2.0 to 10.0 mg/kg.[35]An additional 18 countries have a voluntary fortification program. For example, theIndian governmentrecommends 3.5 mg/kg for"maida" (white)and"atta" (whole wheat)flour.[36]
Synthesis
[edit]Biosynthesis
[edit]Thiamine biosynthesis occurs in bacteria, some protozoans, plants, and fungi.[37][38]Thethiazoleandpyrimidinemoieties are biosynthesized separately and are then combined to form ThMP by the action ofthiamine-phosphate synthase.
The pyrimidine ring system is formed in a reaction catalysed byphosphomethylpyrimidine synthase(ThiC), an enzyme in theradical SAMsuperfamily ofiron–sulfur proteins,which useS-adenosyl methionineas acofactor.[39][40]
The starting material is5-aminoimidazole ribotide,which undergoes arearrangement reactionviaradicalintermediates which incorporate the blue, green and red fragments shown into the product.[41][42]
The thiazole ring is formed in a reaction catalysed bythiazole synthase(EC 2.8.1.10).[39]The ultimate precursors are 1-deoxy-D-xylulose 5-phosphate, 2-iminoacetate and asulfur carrier proteincalled ThiS. An additional protein, ThiG, is also required to bring together all the components of the ring at the enzyme active site.[43]
The final step to form ThMP involvesdecarboxylationof the thiazole intermediate, which reacts with thepyrophosphatederivative of phosphomethylpyrimidine, itself a product of akinase,phosphomethylpyrimidine kinase.[39]
The biosynthetic pathways differ among organisms. InE. coliand otherenterobacteriaceae,ThMP is phosphorylated to the cofactor TPP by athiamine-phosphate kinase(ThMP + ATP → TPP + ADP).[39]In most bacteria and ineukaryotes,ThMP is hydrolyzed to thiamine and then pyrophosphorylated to TPP bythiamine diphosphokinase(thiamine + ATP → TPP + AMP).[44]
The biosynthetic pathways are regulated byriboswitches.[3]If there is sufficient thiamine present in the cell then the thiamine binds to themRNAsfor the enzymes that are required in the pathway and prevents theirtranslation.If there is no thiamine present then there is no inhibition, and the enzymes required for the biosynthesis are produced. The specific riboswitch, theTPP riboswitch,is the only known riboswitch found in both eukaryotic andprokaryoticorganisms.[45]
Laboratory synthesis
[edit]In the firsttotal synthesisin 1936, ethyl 3-ethoxypropanoate was treated withethyl formateto give an intermediate dicarbonyl compound which when reacted withacetamidineformed a substitutedpyrimidine.Conversion of its hydroxyl group to an amino group was carried out bynucleophilic aromatic substitution,first to the chloride derivative usingphosphorus oxychloride,followed by treatment withammonia.Theethoxygroup was then converted to a bromo derivative usinghydrobromic acid.In the final stage, thiamine (as its dibromide salt) was formed in analkylationreaction using 4-methyl-5-(2-hydroxyethyl)thiazole.[46]: 7 [47]
Industrial synthesis
[edit]
Merck & Co.adapted the 1936 laboratory-scale synthesis, allowing them to manufacture thiamine inRahwayin 1937.[47]However, an alternative route using the intermediate Grewediamine(5-(aminomethyl)-2-methyl-4-pyrimidinamine), first published in 1937,[48]was investigated byHoffman La Rocheand competitive manufacturing processes followed. Efficient routes to the diamine have continued to be of interest.[47][49]In theEuropean Economic Area,thiamine is registered underREACH regulationand between 100 and 1,000 tonnes per annum are manufactured or imported there.[50]
Synthetic analogues
[edit]Manyvitamin B1analogues,such asBenfotiamine,fursultiamine,andsulbutiamine,are synthetic derivatives of thiamine. Most were developed in Japan in the 1950s and 1960s as forms that were intended to improve absorption compared to thiamine.[51]Some are approved for use in some countries as a drug or non-prescription dietary supplement for treatment ofdiabetic neuropathyor other health conditions.[52][53][54]
Absorption, metabolism and excretion
[edit]In the upper small intestine, thiamine phosphate esters present in food are hydrolyzed by alkalinephosphataseenzymes. At low concentrations, the absorption process is carrier-mediated. At higher concentrations, absorption also occurs viapassive diffusion.[3]Active transport can be inhibited by alcohol consumption or byfolate deficiency.[10]
The majority of thiamine inserumis bound to proteins, mainlyalbumin.Approximately90% of total thiamine in blood is inerythrocytes.A specific binding protein called thiamine-binding protein has been identified in rat serum and is believed to be a hormone-regulated carrier protein important for tissue distribution of thiamine.[14]Uptake of thiamine by cells of the blood and other tissues occurs via active transport and passive diffusion.[10]Two members of the family of transporter proteins encoded by the genesSLC19A2andSLC19A3are capable of thiamine transport.[20]In some tissues, thiamine uptake and secretion appear to be mediated by a Na+-dependent transporter and a transcellular proton gradient.[14]
Human storage of thiamine is about 25 to 30 mg, with the greatest concentrations in skeletal muscle, heart, brain, liver, and kidneys. ThMP and free (unphosphorylated) thiamine are present in plasma, milk,cerebrospinal fluid,and, it is presumed, allextracellular fluid.Unlike the highly phosphorylated forms of thiamine, ThMP and free thiamine are capable of crossing cell membranes. Calcium and magnesium have been shown to affect the distribution of thiamine in the body andmagnesium deficiencyhas been shown to aggravate thiamine deficiency.[20]Thiamine contents in human tissues are less than those of other species.[14][55]
Thiamine and its metabolites (2-methyl-4-amino-5-pyrimidine carboxylic acid, 4-methyl-thiazole-5-acetic acid, and others) are excreted principally in the urine.[3]
Interference
[edit]Thebioavailabilityof thiamine in foods can be interfered with in a variety of ways.Sulfites,added to foods as a preservative,[56]will attack thiamine at the methylene bridge, cleaving the pyrimidine ring from the thiazole ring. The rate of this reaction is increased under acidic conditions.[14]Thiamine is degraded by thermolabilethiaminasespresent in some species of fish, shellfish and other foods.[10]The pupae of an African silk worm,Anaphe venata,is a traditional food in Nigeria. Consumption leads to thiamine deficiency.[57]Older literature reported that in Thailand, consumption of fermented, uncooked fish caused thiamine deficiency, but either abstaining from eating the fish or heating it first reversed the deficiency.[58]In ruminants, intestinal bacteria synthesize thiamine and thiaminases. The bacterial thiaminases are cell surface enzymes that must dissociate from the cell membrane before being activated; the dissociation can occur in ruminants underacidotic conditions.Indairy cows,over-feeding with grain causes subacute ruminal acidosis and increased ruminal bacteria thiaminase release, resulting in thiamine deficiency.[59]
From reports on two small studies conducted in Thailand, chewing slices ofareca nutwrapped inbetelleaves and chewing tea leaves reduced food thiamine bioavailability by a mechanism that may involvetannins.[58][60]
Bariatric surgery for weight loss is known to interfere with vitamin absorption.[61]A meta-analysis reported that27% of people who underwent bariatric surgeries experience vitamin B1deficiency.[62]
History
[edit]Thiamine was the first of the water-soluble vitamins to be isolated.[63]The earliest observations in humans and in chickens had shown that diets of primarily polished white rice caused beriberi, but did not attribute it to the absence of a previously unknown essential nutrient.[64][65]
In 1884,Takaki Kanehiro,a surgeon general in theImperial Japanese Navy,rejected the previousgerm theoryfor beriberi and suggested instead that the disease was due to insufficiencies in the diet.[64]Switching diets on a navy ship, he discovered that replacing a diet of white rice only with one also containing barley, meat, milk, bread, and vegetables, nearly eliminated beriberi on a nine-month sea voyage. However, Takaki had added many foods to the successful diet and he incorrectly attributed the benefit to increased protein intake, as vitamins were unknown at the time. The Navy was not convinced of the need for such an expensive program of dietary improvement, and many men continued to die of beriberi, even during theRusso-Japanese warof 1904–5. Not until 1905, after the anti-beriberi factor had been discovered inrice bran(removed bypolishing into white rice) and in barley bran, was Takaki's experiment rewarded. He was made a baron in the Japanese peerage system, after which he was affectionately called "Barley Baron".[64]
The specific connection to grain was made in 1897 byChristiaan Eijkman,a military doctor in theDutch East Indies,who discovered that fowl fed on a diet of cooked, polished rice developed paralysis that could be reversed by discontinuing rice polishing.[65]He attributed beriberi to the high levels ofstarchin rice being toxic. He believed that the toxicity was countered in a compound present in the rice polishings.[66]An associate,Gerrit Grijns,correctly interpreted the connection between excessive consumption of polished rice and beriberi in 1901: He concluded that rice contains an essential nutrient in the outer layers of the grain that is removed by polishing.[67]Eijkman was eventually awarded theNobel Prize in Physiology and Medicinein 1929, because his observations led to the discovery of vitamins.
In 1910, a Japanese agricultural chemist ofTokyo Imperial University,Umetaro Suzuki,isolated a water-soluble thiamine compound from rice bran, which he namedaberic acid.(He later renamed itOrizanin.) He described the compound as not only an anti-beriberi factor, but also as being essential to human nutrition; however, this finding failed to gain publicity outside of Japan, because a claim that the compound was a new finding was omitted in translation of his publication from Japanese to German.[63]In 1911 a Polish biochemistCasimir Funkisolated theantineuriticsubstance from rice bran (the modern thiamine) that he called a "vitamine" (on account of its containing an amino group).[68][69]However, Funk did not completely characterize its chemical structure. Dutch chemists,Barend Coenraad Petrus Jansenand his closest collaborator Willem Frederik Donath, went on to isolate and crystallize the active agent in 1926,[70]whose structure was determined byRobert Runnels Williams,in 1934. Thiamine was named by the Williams team as aportmanteauof "thio" (meaning sulfur-containing) and "vitamin". The term "vitamin" coming indirectly, by way of Funk, from the amine group of thiamine itself (although by this time, vitamins were known to not always be amines, for example,vitamin C). Thiamine was also synthesized by the Williams group in 1936.[71]
SirRudolph Peters,inOxford,used pigeons to understand how thiamine deficiency results in the pathological-physiological symptoms of beriberi. Pigeons fed exclusively on polished rice developedopisthotonos,a condition characterized by head retraction. If not treated, the animals died after a few days. Administration of thiamine after opisthotonos was observed led to a complete cure within 30 minutes. As no morphological modifications were seen in the brain of the pigeons before and after treatment with thiamine, Peters introduced the concept of a biochemical-induced injury.[72]In 1937, Lohmann and Schuster showed that the diphosphorylated thiamine derivative, TPP, was a cofactor required for the oxidative decarboxylation of pyruvate.[73]
- Some contributors to the discovery of thiamine
References
[edit]- ^abcdef"Thiamin Fact Sheets for Health Professionals".Office of Dietary Supplements.11 February 2016.Archivedfrom the original on 30 December 2016.Retrieved30 December2016.
- ^Smithline HA, Donnino M, Greenblatt DJ (February 2012)."Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects".BMC Clinical Pharmacology.12(1): 4.doi:10.1186/1472-6904-12-4.PMC3293077.PMID22305197.
- ^abcdefgBettendorff L (2020). "Thiamine". In Marriott BP, Birt DF, Stallings VA, Yates AA (eds.).Present Knowledge in Nutrition, Eleventh Edition.London, United Kingdom: Academic Press (Elsevier). pp. 171–88.ISBN978-0-323-66162-1.
- ^abcdefgInstitute of Medicine(1998)."Thiamin".Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline.Washington, DC: The National Academies Press. pp. 58–86.ISBN978-0-309-06554-2.Archivedfrom the original on 16 July 2015.Retrieved29 August2017.
- ^"Thiamine: MedlinePlus Drug Information".medlineplus.gov.Archivedfrom the original on 28 April 2018.Retrieved30 April2018.
- ^ab"Thiamin".Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2013.Archivedfrom the original on 2 February 2017.Retrieved2 February2022.
- ^abcdAmerican Society of Health-System Pharmacists."Thiamine Hydrochloride".Drugsite Trust (Drugs.com).Archivedfrom the original on 9 August 2020.Retrieved17 April2018.
- ^Kliegman RM, Stanton B (2016).Nelson Textbook of Pediatrics.Elsevier Health Sciences. p. 322.ISBN9781455775668.
There are no cases of adverse effects of excess thiamine... A few isolated cases of puritis...
- ^World Health Organization(2019).World Health Organization model list of essential medicines: 21st list 2019.Geneva: World Health Organization.hdl:10665/325771.WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^abcdefgMahan LK, Escott-Stump S, eds. (2000).Krause's food, nutrition, & diet therapy(10th ed.). Philadelphia: W.B. Saunders Company.ISBN978-0-7216-7904-4.
- ^abcButterworth RF (2006). "Thiamin". In Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds.).Modern Nutrition in Health and Disease(10th ed.). Baltimore: Lippincott Williams & Wilkins.
- ^Bettendorff L, Wins P (2013). "Biochemistry of Thiamine and Thiamine Phosphate Compounds".Encyclopedia of Biological Chemistry.pp. 202–9.doi:10.1016/B978-0-12-378630-2.00102-X.ISBN9780123786319.
- ^McCandless D (2010).Thiamine Deficiency and Associate Clinical Disorders.New York, NY: Humana Press. pp. 157–9.ISBN978-1-60761-310-7.
- ^abcdeCombs Jr GF (2008).The Vitamins: Fundamental Aspects in Nutrition and Health(3rd ed.). Ithaca, NY: Elsevier Academic Press.ISBN978-0-12-183493-7.
- ^Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, Mynak ML, Fischer PR (August 2021)."Thiamine deficiency disorders: a clinical perspective".Annals of the New York Academy of Sciences.1498(1): 9–28.Bibcode:2021NYASA1498....9S.doi:10.1111/nyas.14536.PMC8451766.PMID33305487.
- ^Katta N, Balla S, Alpert MA (July 2016)."Does Long-Term Furosemide Therapy Cause Thiamine Deficiency in Patients with Heart Failure? A Focused Review".The American Journal of Medicine.129(7): 753.e7–753.e11.doi:10.1016/j.amjmed.2016.01.037.PMID26899752.
- ^Gomes F, Bergeron G, Bourassa MW, Fischer PR (August 2021)."Thiamine deficiency unrelated to alcohol consumption in high-income countries: a literature review".Annals of the New York Academy of Sciences.1498(1): 46–56.Bibcode:2021NYASA1498...46G.doi:10.1111/nyas.14569.PMC8451800.PMID33576090.
- ^abFitzpatrick TB, Chapman LM (August 2020)."The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification ".The Journal of Biological Chemistry.295(34): 12002–13.doi:10.1074/jbc.REV120.010918.PMC7443482.PMID32554808.
- ^Mkrtchyan G, Aleshin V, Parkhomenko Y, Kaehne T, Di Salvo ML, Parroni A, et al. (July 2015)."Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis".Scientific Reports.5:12583.Bibcode:2015NatSR...512583M.doi:10.1038/srep12583.PMC4515825.PMID26212886.
- ^abcdeLonsdale D (March 2006)."A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives".Evidence-Based Complementary and Alternative Medicine.3(1): 49–59.doi:10.1093/ecam/nek009.PMC1375232.PMID16550223.
- ^Boluda CJ, Juncá C, Soto E, de la Cruz D, Peña A (13 December 2019)."Umpolung in reactions catalyzed by thiamine pyrophosphate dependent enzymes".Ciencia, Ambiente y Clima(in Spanish).2(2): 27–42.doi:10.22206/cac.2019.v2i2.pp27-42.ISSN2636-2333.S2CID213836801.Archivedfrom the original on 1 December 2022.Retrieved1 December2022.
- ^Ciszak EM, Korotchkina LG, Dominiak PM, Sidhu S, Patel MS (June 2003)."Structural basis for flip-flop action of thiamin pyrophosphate-dependent enzymes revealed by human pyruvate dehydrogenase".The Journal of Biological Chemistry.278(23): 21240–21246.doi:10.1074/jbc.M300339200.hdl:2060/20030106063.PMID12651851.
- ^Makarchikov AF, Lakaye B, Gulyai IE, Czerniecki J, Coumans B, et al. (July 2003)."Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals".Cellular and Molecular Life Sciences.60(7): 1477–88.doi:10.1007/s00018-003-3098-4.PMC11146050.PMID12943234.S2CID25400487.
- ^abcBettendorff L (November 2021)."Update on Thiamine Triphosphorylated Derivatives and Metabolizing Enzymatic Complexes".Biomolecules.11(11): 1645.doi:10.3390/biom11111645.PMC8615392.PMID34827643.
- ^Oudman E, Wijnia JW, Oey M, van Dam M, Painter RC, Postma A (May 2019). "Wernicke's encephalopathy in hyperemesis gravidarum: A systematic review".European Journal of Obstetrics, Gynecology, and Reproductive Biology.236:84–93.doi:10.1016/j.ejogrb.2019.03.006.hdl:1874/379566.PMID30889425.S2CID84184482.
- ^Butterworth RF (December 2001)."Maternal thiamine deficiency: still a problem in some world communities".The American Journal of Clinical Nutrition.74(6): 712–3.doi:10.1093/ajcn/74.6.712.PMID11722950.
- ^Kloss O, Eskin NA, Suh M (April 2018). "Thiamin deficiency on fetal brain development with and without prenatal alcohol exposure".Biochemistry and Cell Biology.96(2): 169–77.doi:10.1139/bcb-2017-0082.hdl:1807/87775.PMID28915355.
- ^abcTolerable Upper Intake Levels For Vitamins And Minerals(PDF),European Food Safety Authority, 2006,archived(PDF)from the original on 16 March 2016
- ^"Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies"(PDF).2017.Archived(PDF)from the original on 28 August 2017.
- ^"Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982"(PDF).Archived(PDF)from the original on 8 August 2016.
- ^"Daily Value Reference of the Dietary Supplement Label Database (DSLD)".Dietary Supplement Label Database (DSLD).Archived fromthe originalon 7 April 2020.Retrieved6 February2022.
- ^"Thiamin content per 100 grams; select food subset, abridged list by food groups".United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. 17 January 2017. Archived fromthe originalon 2 February 2017.Retrieved27 January2017.
- ^Kominiarek MA, Rajan P (November 2016)."Nutrition Recommendations in Pregnancy and Lactation".The Medical Clinics of North America.100(6): 1199–215.doi:10.1016/j.mcna.2016.06.004.PMC5104202.PMID27745590.
- ^"What nutrients are added to flour and rice in fortification?".Food Fortification Initiative. 2021.Archivedfrom the original on 8 October 2021.Retrieved8 October2021.
- ^"Map: Count of Nutrients In Fortification Standards".Global Fortification Data Exchange.Archivedfrom the original on 11 April 2019.Retrieved11 October2021.
- ^"Direction under Section 16(5) of Foods Safety and Standards Act, 2006 regarding Operationalisation of Food Safety & Standards (Fortification of Foods) Regulations, 2017 relating to standards for fortification of food"(PDF).Food Safety & Standards Authority of India (FSSAI).19 May 2017.Archived(PDF)from the original on 17 December 2021.Retrieved1 February2022.
- ^Webb ME, Marquet A, Mendel RR, Rébeillé F, Smith AG (October 2007). "Elucidating biosynthetic pathways for vitamins and cofactors".Natural Product Reports.24(5): 988–1008.doi:10.1039/b703105j.PMID17898894.
- ^Begley TP, Chatterjee A, Hanes JW, Hazra A, Ealick SE (April 2008)."Cofactor biosynthesis--still yielding fascinating new biological chemistry".Current Opinion in Chemical Biology.12(2): 118–25.doi:10.1016/j.cbpa.2008.02.006.PMC2677635.PMID18314013.
- ^abcdCaspi R (14 September 2011)."Pathway: superpathway of thiamine diphosphate biosynthesis I".MetaCyc Metabolic Pathway Database.Archivedfrom the original on 1 February 2022.Retrieved1 February2022.
- ^Holliday GL, Akiva E, Meng EC, Brown SD, Calhoun S, et al. (2018). "Atlas of the Radical SAM Superfamily: Divergent Evolution of Function Using a" Plug and Play "Domain".Radical SAM Enzymes.Methods in Enzymology. Vol. 606. pp. 1–71.doi:10.1016/bs.mie.2018.06.004.ISBN9780128127940.PMC6445391.PMID30097089.
- ^Chatterjee A, Hazra AB, Abdelwahed S, Hilmey DG, Begley TP (November 2010)."A" radical dance "in thiamin biosynthesis: mechanistic analysis of the bacterial hydroxymethylpyrimidine phosphate synthase".Angewandte Chemie.49(46): 8653–6.doi:10.1002/anie.201003419.PMC3147014.PMID20886485.
- ^Mehta AP, Abdelwahed SH, Fenwick MK, Hazra AB, Taga ME, Zhang Y, et al. (August 2015)."Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B₁₂ Biosynthesis".Journal of the American Chemical Society.137(33): 10444–7.doi:10.1021/jacs.5b03576.PMC4753784.PMID26237670.
- ^Begley TP (February 2006). "Cofactor biosynthesis: an organic chemist's treasure trove".Natural Product Reports.23(1): 15–25.doi:10.1039/b207131m.PMID16453030.
- ^Caspi R (23 September 2011)."Pathway: superpathway of thiamine diphosphate biosynthesis III (eukaryotes)".MetaCyc Metabolic Pathway Database.Archivedfrom the original on 14 November 2022.Retrieved14 November2022.
- ^Bocobza SE, Aharoni A (October 2008). "Switching the light on plant riboswitches".Trends in Plant Science.13(10): 526–33.Bibcode:2008TPS....13..526B.doi:10.1016/j.tplants.2008.07.004.PMID18778966.
- ^Tylicki A, Łotowski Z, Siemieniuk M, Ratkiewicz A (February 2018)."Thiamine and selected thiamine antivitamins - biological activity and methods of synthesis".Bioscience Reports.38(1).doi:10.1042/BSR20171148.PMC6435462.PMID29208764.
- ^abcEggersdorfer M, Laudert D, Létinois U, McClymont T, Medlock J, Netscher T, Bonrath W (December 2012). "One hundred years of vitamins-a success story of the natural sciences".Angewandte Chemie.51(52): 12960–90.doi:10.1002/anie.201205886.PMID23208776.
- ^Todd AR, Bergel F (1937). "73. Aneurin. Part VII. A synthesis of aneurin".Journal of the Chemical Society (Resumed):364.doi:10.1039/JR9370000364.
- ^Jiang M, Liu M, Huang H, Chen F (2021). "Fully Continuous Flow Synthesis of 5-(Aminomethyl)-2-methylpyrimidin-4-amine: A Key Intermediate of Vitamin B1".Organic Process Research & Development.25(10): 2331–7.doi:10.1021/acs.oprd.1c00253.S2CID242772232.
- ^"Substance Infocard".echa.europa.eu.Archivedfrom the original on 20 April 2021.Retrieved11 May2022.
- ^Bettendorff L (2014). "Chapter 7 - Thiamine". In Zempleni J, Suttie JW, Gregory JF, Stover PJ (eds.).Handbook of vitamins(5th ed.). Hoboken: CRC Press. pp. 267–324.ISBN9781466515574.
- ^Zaheer A, Zaheer F, Saeed H, Tahir Z, Tahir MW (April 2021)."A Review of Alternative Treatment Options in Diabetic Polyneuropathy".Cureus.13(4). e14600.doi:10.7759/cureus.14600.PMC8139599.PMID34040901.
- ^McCarty MF, Inoguchi T (2008)."11. Targeting Oxidant Stress as a Strategy for Preventing Vascular Complications of Diabetes and Metabolic Syndrome".In Pasupuleti VK, Anderson JW (eds.).Nutraceuticals, glycemic health and type 2 diabetes(1st ed.). Ames, Iowa: Wiley-Blackwell/IFT Press. p. 213.ISBN9780813804286.
- ^Lonsdale D (September 2004)."Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent".Medical Science Monitor.10(9): RA199–203.PMID15328496.Archivedfrom the original on 25 September 2012.Retrieved17 July2022.
- ^Bettendorff L, Mastrogiacomo F, Kish SJ, Grisar T (January 1996). "Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain".Journal of Neurochemistry.66(1): 250–8.doi:10.1046/j.1471-4159.1996.66010250.x.PMID8522961.S2CID7161882.
- ^McGuire M, Beerman KA (2007).Nutritional Sciences: From Fundamentals to Foods.California: Thomas Wadsworth.
- ^Nishimune T, Watanabe Y, Okazaki H, Akai H (2000)."Thiamin is decomposed due to Anaphe spp. entomophagy in seasonal ataxia patients in Nigeria".J. Nutr.130(6): 1625–8.doi:10.1093/jn/130.6.1625.PMID10827220.
- ^abVimokesant SL, Hilker DM, Nakornchai S, Rungruangsak K, Dhanamitta S (December 1975). "Effects of betel nut and fermented fish on the thiamin status of northeastern Thais".Am J Clin Nutr.28(12): 1458–63.doi:10.1093/ajcn/28.12.1458.PMID803009.
- ^Pan X, Nan X, Yang L, Jiang L, Xiong B (September 2018)."Thiamine status, metabolism and application in dairy cows: a review".Br J Nutr.120(5): 491–9.doi:10.1017/S0007114518001666.PMID29986774.S2CID51606809.
- ^Vimokesant S, Kunjara S, Rungruangsak K, Nakornchai S, Panijpan B (1982). "Beriberi caused by antithiamin factors in food and its prevention".Ann N Y Acad Sci.378(1): 123–36.Bibcode:1982NYASA.378..123V.doi:10.1111/j.1749-6632.1982.tb31191.x.PMID7044221.S2CID40854060.
- ^Nunes R, Santos-Sousa H, Vieira S, Nogueiro J, Bouça-Machado R, et al. (March 2022). "Vitamin B Complex Deficiency After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy-a Systematic Review and Meta-Analysis".Obes Surg.32(3): 873–91.doi:10.1007/s11695-021-05783-2.PMID34982396.S2CID245655046.
- ^Bahardoust M, Eghbali F, Shahmiri SS, Alijanpour A, Yarigholi F, et al. (September 2022). "B1 Vitamin Deficiency After Bariatric Surgery, Prevalence, and Symptoms: a Systematic Review and Meta-analysis".Obes Surg.32(9): 3104–12.doi:10.1007/s11695-022-06178-7.PMID35776243.S2CID250149680.
- ^abSuzuki U, Shimamura T (1911)."Active constituent of rice grits preventing bird polyneuritis".Tokyo Kagaku Kaishi.32:4–7, 144–6, 335–58.doi:10.1246/nikkashi1880.32.4.Archivedfrom the original on 21 June 2020.Retrieved2 May2018.
- ^abcMcCollum EV (1957).A History of Nutrition.Cambridge, Massachusetts: Riverside Press,Houghton Mifflin.
- ^abEijkman C (1897)."Eine Beriberiähnliche Krankheit der Hühner"[A disease of chickens which is similar to beriberi].Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin.148(3): 523–532.doi:10.1007/BF01937576.S2CID38445999.Archivedfrom the original on 9 August 2020.Retrieved4 July2019.
- ^"The Nobel Prize and the Discovery of Vitamins".nobelprize.org.Archivedfrom the original on 16 January 2018.Retrieved1 May2018.
- ^Grijns G (1901)."Over polyneuritis gallinarum"[On polyneuritis gallinarum].Geneeskundig Tijdschrift voor Nederlandsch-Indië (Medical Journal for the Dutch East Indies).41(1): 3–11.Archivedfrom the original on 29 August 2021.Retrieved5 February2020.
- ^Funk C (December 1911)."On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice".The Journal of Physiology.43(5): 395–400.doi:10.1113/jphysiol.1911.sp001481.PMC1512869.PMID16993097.
- ^Funk C (1912)."The etiology of the deficiency diseases. Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra".Journal of State Medicine.20:341–68.Archivedfrom the original on 4 July 2020.Retrieved5 February2020.The word "vitamine" is coined on p. 342: "It is now known that all these diseases, with the exception of pellagra, can be prevented and cured by the addition of certain preventative substances; the deficient substances, which are of the nature of organic bases, we will call" vitamines "; and we will speak of a beri-beri or scurvy vitamine, which means a substance preventing the special disease."
- ^Jansen BC, Donath WF (1926). "On the isolation of antiberiberi vitamin".Proc. Kon. Ned. Akad. Wet.29:1390–400.
- ^Williams RR, Cline JK (1936). "Synthesis of Vitamin B1".Journal of the American Chemical Society.58(8): 1504–5.doi:10.1021/ja01299a505.
- ^Peters RA (1936). "The biochemical lesion in vitamin B1deficiency. Application of modern biochemical analysis in its diagnosis ".Lancet.230(5882): 1161–4.doi:10.1016/S0140-6736(01)28025-8.
- ^Lohmann K, Schuster P (1937). "Untersuchungen über die Cocarboxylase".Biochem. Z.294:188–214.
External links
[edit]- "Thiamine".Drug Information Portal.US National Library of Medicine.