Y chromosome
| Human Y chromosome | |
|---|---|
Human Y chromosome (afterG-banding) | |
 Y chromosome in human malekaryogram | |
| Features | |
| Length (bp) | 62,460,029 bp (CHM13) |
| No.of genes | 63 (CCDS)[1] |
| Type | Allosome |
| Centromere position | Acrocentric[2] (10.4 Mbp[3]) |
| Complete gene lists | |
| CCDS | Gene list |
| HGNC | Gene list |
| UniProt | Gene list |
| NCBI | Gene list |
| External map viewers | |
| Ensembl | Chromosome Y |
| Entrez | Chromosome Y |
| NCBI | Chromosome Y |
| UCSC | Chromosome Y |
| Full DNA sequences | |
| RefSeq | NC_000024(FASTA) |
| GenBank | CM000686(FASTA) |
TheY chromosomeis one of twosex chromosomesintherian mammalsandother organisms.Along with theX chromosome,it is part of theXY sex-determination system,in which the Y is thesex-determiningbecause it is the presence or absence of Y chromosome that determines the male or femalesexofoffspringproduced insexual reproduction.In mammals, the Y chromosome contains theSRYgene, which triggers development ofmale gonads.The Y chromosome is passed only from male parents to male offspring.
Overview[edit]
Discovery[edit]
The Y chromosome was identified as a sex-determining chromosome byNettie StevensatBryn Mawr Collegein 1905 during a study of themealwormTenebrio molitor.Edmund Beecher Wilsonindependently discovered the same mechanisms the same year, working withHemiptera.Stevens proposed that chromosomes always existed in pairs and that the smaller chromosome (now labelled "Y" ) was the pair of the X chromosome discovered in 1890 byHermann Henking.She realized that the previous idea ofClarence Erwin McClung,that the X chromosome determines sex, was wrong and thatsex determinationis, in fact, due to the presence or absence of the Y chromosome. In the early 1920sTheophilus Painterdetermined that X and Y chromosomes determined sex in humans (and other mammals).[4]
The chromosome was given the name "Y" simply to follow on from Henking's "X" alphabetically.[5][6]The idea that the Y chromosome was named after its similarity in appearance to the letter "Y" is mistaken. All chromosomes normally appear as an amorphous blob under the microscope and only take on a well-defined shape duringmitosis.This shape is vaguely X-shaped for all chromosomes. It is entirely coincidental that the Y chromosome, duringmitosis,has two very short branches which can look merged under the microscope and appear as the descender of a Y-shape.[5]: 65–66
Variations[edit]
Most therian mammals have only one pair of sex chromosomes in each cell. Males have one Y chromosome and oneX chromosome,while females have two X chromosomes. In mammals, the Y chromosome contains a gene,SRY,which triggers embryonic development as a male. The Y chromosomes of humans and other mammals also contain other genes needed for normal sperm production.[citation needed]
There are exceptions, however. Among humans, some males are born two Xs and a Y ( "XXY", seeKlinefelter syndrome), one X and two Ys (seeXYY syndrome). Some females have three Xs (Trisomy X), and some have a single X instead of two Xs ( "X0", seeTurner syndrome). There are other variations in which, duringembryonic development,theWNT4gene[7]is activated and/or the SRY gene is damaged leading to birth of anXY female(Swyer syndrome[7]). A Y chromosome may also be present but fail to result in the development of a male phenotype in individuals withandrogen insensitivity syndrome,instead resulting in a female or ambiguous phenotype. In other cases, the SRY gene is copied to the X, leading to birth of anXX male.[8]
Origins and evolution[edit]
Before Y chromosome[edit]
Manyectothermicvertebrateshave no sex chromosomes.[9]If these species have different sexes, sex is determined environmentally rather than genetically. For some species, especiallyreptiles,sex depends on the incubation temperature.[10]Some vertebrates arehermaphrodites,though hermaphroditic species are most commonlysequential,meaning the organism switches sex, producing male or femalegametesat different points in its life, but never producing both at the same time. This is opposed tosimultaneoushermaphroditism, where the same organism produces male and female gametes at the same time. Most simultaneous hermaphrodite species are invertebrates, and among vertebrates, simultaneous hermaphroditism has only been discovered in a fewordersof fish.[11]
Origin[edit]
The X and Y chromosomes are thought to have evolved from a pair of identical chromosomes,[12][13]termedautosomes,when an ancestral animal developed an allelic variation (a so-called "sex locus" ) and simply possessing thisallelecaused the organism to be male.[14]The chromosome with this allele became the Y chromosome, while the other member of the pair became the X chromosome. Over time, genes that were beneficial for males and harmful to (or had no effect on) females either developed on the Y chromosome or were acquired by the Y chromosome through the process oftranslocation.[15]
Until recently, the X and Y chromosomes were thought to have diverged around 300 million years ago.[16]However, research published in 2008 analyzing theplatypusgenome[17]suggested that the XY sex-determination system would not have been present more than 166 million years ago, whenmonotremessplit from other mammals.[18]This re-estimation of the age of thetherianXY system is based on the finding that sequences that are on the X chromosomes ofmarsupialsandeutherianmammals are not present on the autosomes of platypus and birds.[18]The older estimate was based on erroneous reports that the platypus X chromosomes contained these sequences.[19][20]
Recombination inhibition[edit]
Most chromosomesrecombineduring meiosis. However, in males, the X and Y pair in a shared region known as thepseudoautosomal region(PAR).[21]The PAR undergoes frequent recombination between the X and Y chromosomes,[21]but recombination is suppressed in other regions of the Y chromosome.[14]These regions contain sex-determining and other male-specific genes.[22]Without this suppression, these genes could be lost from the Y chromosome from recombination and cause issues such as infertility.[23]
The lack of recombination across the majority of the Y chromosome makes it a useful tool in studyinghuman evolution,since recombination complicates the mathematical models used to trace ancestries.[24]
Degeneration[edit]
By one estimate, the human Y chromosome has lost 1,393 of its 1,438 original genes over the course of its existence, andlinear extrapolationof this 1,393-gene loss over 300 million years gives a rate of genetic loss of 4.6 genes per million years.[25]Continued loss of genes at the rate of 4.6 genes per million years would result in a Y chromosome with no functional genes – that is the Y chromosome would lose complete function – within the next 10 million years, or half that time with the current age estimate of 160 million years.[14][26]Comparative genomicanalysis reveals that many mammalian species are experiencing a similar loss of function in their heterozygous sex chromosome. Degeneration may simply be the fate of all non-recombining sex chromosomes, due to three common evolutionary forces: highmutation rate,inefficientselection,andgenetic drift.[14]
With a 30% difference between humans and chimpanzees, the Y chromosome is one of the fastest-evolving parts of thehuman genome.[27]However, these changes have been limited to non-coding sequences and comparisons of the human andchimpanzeeY chromosomes (first published in 2005) show that the human Y chromosome has not lost any genes since the divergence of humans and chimpanzees between 6–7 million years ago.[28]Additionally, a scientific report in 2012 stated that only one gene had been lost since humans diverged from therhesus macaque25 million years ago.[29]These facts provide direct evidence that thelinear extrapolationmodel is flawed and suggest that the current human Y chromosome is either no longer shrinking or is shrinking at a much slower rate than the 4.6 genes per million years estimated by the linear extrapolation model.[citation needed]
High mutation rate[edit]
The human Y chromosome is particularly exposed to high mutation rates due to the environment in which it is housed. The Y chromosome is passed exclusively throughsperm,which undergo multiplecell divisionsduringgametogenesis.Each cellular division provides further opportunity to accumulate base pair mutations. Additionally, sperm are stored in the highly oxidative environment of thetestis,which encourages further mutation. These two conditions combined put the Y chromosome at a greater opportunity of mutation than the rest of the genome.[14]The increased mutation opportunity for the Y chromosome is reported by Graves as a factor 4.8.[14]However, her original reference obtains this number for the relative mutation rates in male and female germ lines for the lineage leading to humans.[30]
The observation that the Y chromosome experiences littlemeioticrecombinationand has an accelerated rate ofmutationand degradative change compared to the rest of thegenomesuggests an evolutionary explanation for the adaptive function ofmeiosiswith respect to the main body of genetic information. Brandeis[31]proposed that the basic function of meiosis (particularly meiotic recombination) is the conservation of the integrity of the genome, a proposal consistent with the idea that meiosis is an adaptation forrepairing DNA damage.[32]
Inefficient selection[edit]
Without the ability to recombine duringmeiosis,the Y chromosome is unable to expose individualallelesto natural selection. Deleterious alleles are allowed to "hitchhike" with beneficial neighbors, thus propagating maladapted alleles into the next generation. Conversely, advantageous alleles may be selected against if they are surrounded by harmful alleles (background selection). Due to this inability to sort through its gene content, the Y chromosome is particularly prone to the accumulation of"junk" DNA.Massive accumulations of retrotransposable elements are scattered throughout the Y.[14]The random insertion of DNA segments often disrupts encoded gene sequences and renders them nonfunctional. However, the Y chromosome has no way of weeding out these "jumping genes". Without the ability to isolate alleles, selection cannot effectively act upon them.[citation needed]
A clear, quantitative indication of this inefficiency is theentropy rateof the Y chromosome. Whereas all other chromosomes in thehuman genomehave entropy rates of 1.5–1.9 bits per nucleotide (compared to the theoretical maximum of exactly 2 for no redundancy), the Y chromosome's entropy rate is only 0.84.[33]This means the Y chromosome has a much lower information content relative to its overall length; it is more redundant.[citation needed]
Genetic drift[edit]
Even if a well adapted Y chromosome manages to maintain genetic activity by avoiding mutation accumulation, there is no guarantee it will be passed down to the next generation. The population size of the Y chromosome is inherently limited to 1/4 that of autosomes: diploid organisms contain two copies of autosomal chromosomes while only half the population contains 1 Y chromosome. Thus, genetic drift is an exceptionally strong force acting upon the Y chromosome. Through sheer random assortment, an adult male may never pass on his Y chromosome if he only has female offspring. Thus, although a male may have a well adapted Y chromosome free of excessive mutation, it may never make it into the next gene pool.[14]The repeat random loss of well-adapted Y chromosomes, coupled with the tendency of the Y chromosome to evolve to have more deleterious mutations rather than less for reasons described above, contributes to the species-wide degeneration of Y chromosomes throughMuller's ratchet.[34]
Gene conversion[edit]
As it has been already mentioned, the Y chromosome is unable to recombine duringmeiosislike the other human chromosomes; however, in 2003, researchers fromMITdiscovered a process which may slow down the process of degradation. They found that human Y chromosome is able to "recombine" with itself, usingpalindromebase pairsequences.[35]Such a "recombination" is calledgene conversion.
In the case of the Y chromosomes, thepalindromesare notnoncoding DNA;these strings of bases contain functioning genes important for male fertility. Most of the sequence pairs are greater than 99.97% identical. The extensive use of gene conversion may play a role in the ability of the Y chromosome to edit out genetic mistakes and maintain the integrity of the relatively few genes it carries. In other words, since the Y chromosome is single, it has duplicates of its genes on itself instead of having a second, homologous, chromosome. When errors occur, it can use other parts of itself as a template to correct them.[35]
Findings were confirmed by comparing similar regions of the Y chromosome in humans to the Y chromosomes ofchimpanzees,bonobosandgorillas.The comparison demonstrated that the same phenomenon of gene conversion appeared to be at work more than 5 million years ago, when humans and the non-human primates diverged from each other.[35]
Gene conversiontracts formed duringmeiosisare long, about 2,068 base pairs, and significantly biased towards the fixation of G or C nucleotides (GC biased).[36]Therecombinationintermediates preceding gene conversion were found to rarely take the alternate route of crossover recombination.[36]The Y-Y gene conversion rate in humans is about 1.52 x 10-5conversions/base/year.[37]These gene conversion events may reflect a basic function of meiosis, that of conserving the integrity of the genome.
Future evolution[edit]
According to some theories, in the terminal stages of the degeneration of the Y chromosome, other chromosomes may increasingly take over genes and functions formerly associated with it and finally, within the framework of this theory, the Y chromosome disappears entirely, and a new sex-determining system arises.[14][neutralityisdisputed][improper synthesis?]Several species ofrodentin the sister familiesMuridaeandCricetidaehave reached these stages,[38][39]in the following ways:
- TheTranscaucasian mole vole,Ellobius lutescens,theZaisan mole vole,Ellobius tancrei,and the Japanese spinous country ratsTokudaia osimensisandTokudaia tokunoshimensis,have lost the Y chromosome andSRYentirely.[14][40][41]Tokudaiaspp. have relocated some other genes ancestrally present on the Y chromosome to the X chromosome.[41]Both sexes ofTokudaiaspp. andEllobius lutescenshave an XO genotype (Turner syndrome),[41]whereas allEllobius tancreipossess an XX genotype.[14]The new sex-determining system(s) for these rodents remains unclear.
- Thewood lemmingMyopus schisticolor,theArctic lemming,Dicrostonyx torquatus,and multiple species in the grass mouse genusAkodonhave evolved fertile females who possess the genotype generally coding for males, XY, in addition to the ancestral XX female, through a variety of modifications to the X and Y chromosomes.[38][42][43]
- In thecreeping vole,Microtus oregoni,the females, with just one X chromosome each, produce X gametes only, and the males, XY, produce Y gametes, or gametes devoid of any sex chromosome, throughnondisjunction.[44]
Outside of the rodents, theblack muntjac,Muntiacus crinifrons,evolved new X and Y chromosomes through fusions of the ancestral sex chromosomes andautosomes.[45]
Modern data cast doubt on this hypothesis.[16]This conclusion was reached by scientists who studied the Y chromosomes of rhesus monkeys. When genomically comparing the Y chromosome of rhesus monkeys and humans, scientists found very few differences, given that humans and rhesus monkeys diverged 30 million years ago.[46]
Some organisms have lost the Y chromosome. For example, most species of Nematodes. However, in order for the complete elimination of Y to occur, it was necessary to develop an alternative way of determining sex (for example, by determining sex by the ratio of the X chromosome to autosomes), and any genes necessary for male function had to be moved to other chromosomes.[16]In the meantime, modern data demonstrate the complex mechanisms of Y chromosome evolution and the fact that the disappearance of the Y chromosome is not guaranteed.
1:1 sex ratio[edit]
Fisher's principleoutlines why almost all species usingsexual reproductionhave asex ratioof 1:1.W. D. Hamiltongave the following basic explanation in his 1967 paper on "Extraordinary sex ratios",[47]given the condition that males and females cost equal amounts to produce:
- Suppose male births are less common than female.
- A newborn male then has better mating prospects than a newborn female, and therefore can expect to have more offspring.
- Therefore, parents genetically disposed to produce males tend to have more than average numbers of grandchildren born to them.
- Therefore, the genes for male-producing tendencies spread, and male births become more common.
- As the 1:1 sex ratio is approached, the advantage associated with producing males dies away.
- The same reasoning holds if females are substituted for males throughout. Therefore, 1:1 is the equilibrium ratio.
Non-therian Y chromosome[edit]
Many groups of organisms in addition to therian mammals have Y chromosomes, but these Y chromosomes do not share common ancestry with therian Y chromosomes. Such groups include monotremes,Drosophila,some other insects, some fish, some reptiles, and some plants. InDrosophila melanogaster,the Y chromosome does not trigger male development. Instead, sex is determined by the number of X chromosomes. TheD. melanogasterY chromosome does contain genes necessary for male fertility. So XXYD. melanogasterare female, andD. melanogasterwith a single X (X0), are male but sterile. There are some species of Drosophila in which X0 males are both viable and fertile.[citation needed]
ZW chromosomes[edit]
Other organisms have mirror image sex chromosomes: where the homogeneous sex is the male, said to have two Z chromosomes, and the female is the heterogeneous sex with a Z chromosome and a W chromosome.[48]For example, the ZW sex-determination system is found inbirds,snakes,andbutterflies;the females have ZW sex chromosomes, and males have ZZ sex chromosomes.[48][49][50]
Non-inverted Y chromosome[edit]
There are some species, such as theJapanese rice fish,in which the XY system is still developing and cross over between the X and Y is still possible. Because the male specific region is very small and contains no essential genes, it is even possible to artificially induce XX males and YY females to no ill effect.[51]
Multiple XY pairs[edit]
Monotremes likeplatypusespossess four or five pairs of XY sex chromosomes, each pair consisting of sex chromosomes with homologous regions. The chromosomes of neighboring pairs are partially homologous, such that a chain is formed duringmitosis.[19]The first X chromosome in the chain is also partially homologous with the last Y chromosome, indicating that profound rearrangements, some adding new pieces from autosomes, have occurred in history.[52][53]: fig. 5
Platypus sex chromosomes have strong sequence similarity with the avianZ chromosome,(indicating closehomology),[17]and the SRY gene so central to sex-determination in most other mammals is apparently not involved in platypus sex-determination.[18]
Human Y chromosome[edit]
This section mayrequirecleanupto meet Wikipedia'squality standards.The specific problem is:Too many subsections. Article might benefit from moving h3 subsections into h2 sections, if we can somehow reconcile the gap between all therians and humans. "Origins and evolution" section has a human focus, but the discussion does include all therians.(October 2021) |
The human Y chromosome is composed of about 62 millionbase pairsofDNA,making it similar in size tochromosome 19and represents almost 2% of the total DNA in a malecell.[54][55]The human Y chromosome carries 693genes,107 of which areprotein-coding.[56]However, some genes are repeated, making the number of exclusiveprotein-codinggenes just 42.[56]TheConsensus Coding Sequence (CCDS) Projectonly classifies 63 out of 107 genes, though CCDS estimates are often considered lower bounds due to their conservative classification strategy.[57]All single-copy Y-linked genes arehemizygous(present on only one chromosome) except in cases ofaneuploidysuch asXYY syndromeorXXYY syndrome.Traits that are inherited via the Y chromosome are calledY-linkedtraits, or holandric traits (fromAncient Greekὅλοςhólos,"whole" + ἀνδρόςandrós,"male" ).[58]
Sequence of the human Y chromosome[edit]
At the end of theHuman Genome Project(and after many updates) almost half of the Y chromosome remained un-sequenced even in 2021; a different Y chromosome from the HG002 (GM24385) genome was completely sequenced in January 2022 and is included in the new "complete genome" humanreference genomesequence, CHM13.[56]The completesequencingof a human Y chromosome was shown to contain 62,460,029 base pairs and 41 additionalgenes.[56]This added 30 million base pairs,[56]but it was discovered that the Y chromosome can vary a lot in size between individuals, from 45.2 million to 84.9 million base pairs.[59]
Since almost half of the human Y sequence was unknown before 2022, it could not be screened out as contamination in microbial sequencing projects. As a result, the NCBI RefSeq bacterial genome database mistakenly includes some Y chromosome data.[56]
Structure[edit]
This articleis missing informationabout NRY/MSY structure - How there's a huge chunk ofheterochromatinin q, nomenclature of the palindromes andamplicons,TTTY transcripts, etc. Best if we add a figure that mashes together the tops of Colaco 2018 Fig 1 and PMID 12815422 fig 3..(October 2021) |
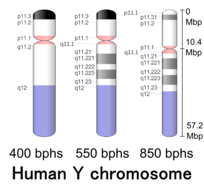
Cytogenetic band[edit]
| Chr. | Arm[64] | Band[65] | ISCN start[66] |
ISCN stop[66] |
Basepair start |
Basepair stop |
Stain[67] | Density |
|---|---|---|---|---|---|---|---|---|
| Y | p | 11.32 | 0 | 149 | 1 | 300,000 | gneg | |
| Y | p | 11.31 | 149 | 298 | 300,001 | 600,000 | gpos | 50 |
| Y | p | 11.2 | 298 | 1043 | 600,001 | 10,300,000 | gneg | |
| Y | p | 11.1 | 1043 | 1117 | 10,300,001 | 10,400,000 | acen | |
| Y | q | 11.1 | 1117 | 1266 | 10,400,001 | 10,600,000 | acen | |
| Y | q | 11.21 | 1266 | 1397 | 10,600,001 | 12,400,000 | gneg | |
| Y | q | 11.221 | 1397 | 1713 | 12,400,001 | 17,100,000 | gpos | 50 |
| Y | q | 11.222 | 1713 | 1881 | 17,100,001 | 19,600,000 | gneg | |
| Y | q | 11.223 | 1881 | 2160 | 19,600,001 | 23,800,000 | gpos | 50 |
| Y | q | 11.23 | 2160 | 2346 | 23,800,001 | 26,600,000 | gneg | |
| Y | q | 12 | 2346 | 3650 | 26,600,001 | 57,227,415 | gvar |
Non-combining region of Y (NRY)[edit]
The human Y chromosome is normally unable to recombine with the X chromosome, except for small pieces ofpseudoautosomal regions(PARs) at thetelomeres(which comprise about 5% of the chromosome's length). These regions are relics of ancienthomologybetween the X and Y chromosomes. The bulk of the Y chromosome, which does not recombine, is called the "NRY", or non-recombining region of the Y chromosome.[68]Single-nucleotide polymorphisms(SNPs) in this region are used to trace direct paternal ancestral lines.
More specifically, PAR1 is at 0.1–2.7 Mb. PAR2 is at 56.9–57.2 Mb. The non-recombining region (NRY) or male-specific region (MSY) sits between. Their sizes is now known perfectly from CHM13: 2.77 Mb and 329.5 kb. Until CHM13 the data in PAR1 and PAR2 was just copied over from X chromosome.[59]
Sequence classes[edit]
Genes[edit]
Number of genes[edit]
The following are some of the gene count estimates of human Y chromosome. Because researchers use different approaches togenome annotationtheir predictions of thenumber of geneson each chromosome varies (for technical details, seegene prediction). Among various projects, CCDS takes an extremely conservative strategy. So CCDS's gene number prediction represents a lower bound on the total number of human protein-coding genes.[69]
| Estimated by | Protein-coding genes | Non-coding RNA genes | Pseudogenes | Source | Release date |
|---|---|---|---|---|---|
| CCDS | 63 | — | — | [1] | 2016-09-08 |
| HGNC | 45 | 55 | 381 | [70] | 2017-05-12 |
| Ensembl | 63 | 109 | 392 | [71] | 2017-03-29 |
| UniProt | 47 | — | — | [72] | 2018-02-28 |
| NCBI | 73 | 122 | 400 | [73][74][75] | 2017-05-19 |
Gene list[edit]
In general, the human Y chromosome is extremely gene poor—it is one of the largestgene desertsin the human genome. Disregardingpseudoautosomalgenes, genes encoded on the human Y chromosome include:
| Name | Xparalog | Note |
|---|---|---|
| SRY | SOX3 | Sex-determining region. This is the p arm [Yp]. |
| ZFY | ZFX | Zinc finger. |
| RPS4Y1 | RPS4X | Ribosomal protein S4. |
| AMELY | AMELX | Amelogenin. |
| TBL1Y | TBL1X | |
| PCDH11Y | PDCH11X | X-transposed region (XTR) from Xq21, one of two genes. Once dubbed "PAR3"[77]but later refuted.[78] |
| TGIF2LY | TGIF2LX | The other X-transposed gene. |
| TSPY1,TSPY2 | TSPX | Testis-specific protein. |
| AZFa | (none) | Not a gene. First part of the AZF (Azoospermia factor) region on arm q. Contains the four following genes. X counterparts escape inactivation. |
| USP9Y | USP9X | Ubiquitin protease. |
| DDX3Y | DDX3X | Helicase. |
| UTY | UTX | Histone demethylase. |
| TB4Y | TB4X | |
| AZFb | (none) | Second AZF region on arm q. Prone to NAHR [non-allelic homologous recombination] with AZFc. Overlaps with AZFc. Contains three single-copy gene regions and repeats. |
| CYorf15 | CXorf15 | |
| RPS4Y2 | RPS4X | Another copy of ribosomal protein S4. |
| EIF1AY | EIF4AX | |
| KDM5D | KDM5C | |
| XKRY | XK (protein) | Found in the "yellow"amplicon. |
| HSFY1,HSFY2 | HSFX1,HSFX2 | Found in the "blue" amplicon. |
| PRY,PRY2 | Found in the "blue" amplicon. Identified by similarity toPTPN13(Chr. 4). | |
| RBMY1A1 | RBMY | Large number of copies. Part of anRBM gene familyof RNA recognition motif (RRM) proteins. |
| AZFc | (none) | Final (distal) part of the AZF. Multiple palindromes. |
| DAZ1,DAZ2,DAZ3,DAZ4 | RRM genes in two palindromic clusters.BOLLandDAZLAare autosomal homologs. | |
| CDY1,CDY2 | CDY1 is actually two identical copies. CDY2 is two closely related copies in palindrome P5. Probably derived from autosomalCDYL. | |
| VCY1,VCY2 | VCX1through 3 | Three copies of VCX2 (BPY2). Part of theVCX/VCYfamily. The two copies of BPY1 are instead in Yq11.221/AZFa. |
Y-chromosome-linked diseases[edit]
Diseases linked to the Y chromosome typically involve ananeuploidy,an atypical number of chromosomes.
Loss of Y chromosome[edit]
Males can lose the Y chromosome in a subset of cells, known asmosaicloss. Mosaic loss is strongly associated with age,[79]and smoking is another important risk factor for mosaic loss.[80]
Mosaic loss may be related to health outcomes, indicating that the Y chromosome plays important roles outside of sex determination.[80][81]Males with a higher percentage ofhematopoieticstem cellslacking the Y chromosome have a higher risk of certaincancersand have a shorter life expectancy.[81]In many cases, a cause and effect relationship between the Y chromosome and health outcomes has not been determined, and some propose loss of the Y chromosome could be a "neutralkaryotyperelated to normalaging".[82]However, a 2022 study showed that mosaic loss of the Y chromosome causally contributes tofibrosis,heart risks,and mortality.[83]
Further studies are needed to understand how mosaic Y chromosome loss may contribute to other sex differences in health outcomes, such as how male smokers have between 1.5 and 2 times the risk of non-respiratory cancers as female smokers.[84][85]Potential countermeasures identified so far include not smoking orstopping smokingand at least one potential drug that "may help counteract the harmful effects of the chromosome loss" is under investigation.[86][87][better source needed]
Y chromosome microdeletion[edit]
Y chromosome microdeletion(YCM) is a family of genetic disorders caused by missing genes in the Y chromosome. Many affected men exhibit no symptoms and lead normal lives. However, YCM is also known to be present in a significant number of men with reduced fertility or reduced sperm count.[citation needed]
Defective Y chromosome[edit]
This results in the person presenting a femalephenotype(i.e., is born with female-like genitalia) even though that person possesses an XYkaryotype.The lack of the second X results in infertility. In other words, viewed from the opposite direction, the person goes throughdefeminizationbut fails to completemasculinization.[citation needed]
The cause can be seen as an incomplete Y chromosome: the usual karyotype in these cases is 45X, plus a fragment of Y. This usually results in defective testicular development, such that the infant may or may not have fully formed male genitalia internally or externally. The full range of ambiguity of structure may occur, especially ifmosaicismis present. When the Y fragment is minimal and nonfunctional, the child is usually a girl with the features ofTurner syndromeormixed gonadal dysgenesis.[citation needed]
XXY[edit]
Klinefelter syndrome (47, XXY) is not ananeuploidyof the Y chromosome, but a condition of having an extra X chromosome, which usually results in defective postnatal testicular function. The mechanism is not fully understood; it does not seem to be due to direct interference by the extra X with expression of Y genes.[citation needed]
XYY[edit]
47, XYY syndrome (simply known as XYY syndrome) is caused by the presence of a single extra copy of the Y chromosome in each of a male's cells. 47, XYY males have one X chromosome and two Y chromosomes, for a total of 47 chromosomes per cell. Researchers have found that an extra copy of the Y chromosome is associated with increased stature and an increased incidence of learning problems in some boys and men, but the effects are variable, often minimal, and the vast majority do not know their karyotype.[88]
In 1965 and 1966Patricia Jacobsand colleagues published a chromosome survey of 315 male patients at Scotland's only special security hospital for thedevelopmentally disabled, finding a higher than expected number of patients to have an extra Y chromosome.[89]The authors of this study wondered "whether an extra Y chromosome predisposes its carriers to unusually aggressive behaviour", and this conjecture "framed the next fifteen years of research on the human Y chromosome".[90]
Through studies over the next decade, this conjecture was shown to be incorrect: the elevated crime rate of XYY males is due to lower median intelligence and not increased aggression,[91]and increased height was the only characteristic that could be reliably associated with XYY males.[92]The "criminal karyotype" concept is therefore inaccurate.[88]
Rare[edit]
The following Y-chromosome-linked diseases are rare, but notable because of their elucidation of the nature of the Y chromosome.
More than two Y chromosomes[edit]
Greater degrees of Y chromosome polysomy (having more than one extra copy of the Y chromosome in every cell, e.g., XYYY) are considerably more rare. The extra genetic material in these cases can lead to skeletal abnormalities, dental abnormalities, decreased IQ, delayed development, and respiratory issues, but the severity features of these conditions are variable.[93]
XX male syndrome[edit]
XX male syndromeoccurs due to agenetic recombinationin the formation of the malegametes,causing theSRYportion of the Y chromosome to move to the X chromosome.[8]When such an X chromosome is present in a zygote, male gonads develop because of the SRY gene.[8]
Genetic genealogy[edit]
In humangenetic genealogy(the application ofgeneticstotraditional genealogy), use of the information contained in the Y chromosome is of particular interest because, unlike other chromosomes, the Y chromosome is passed exclusively from father to son, on the patrilineal line.Mitochondrial DNA,maternally inherited to both sons and daughters, is used in an analogous way to trace the matrilineal line.[citation needed]
Brain function[edit]
Research is currently investigating whether male-pattern neural development is a direct consequence of Y-chromosome-related gene expression or an indirect result of Y-chromosome-relatedandrogenic hormoneproduction.[94]
Microchimerism[edit]
In 1974, male chromosomes were discovered in fetal cells in the blood circulation of women.[95]
In 1996, it was found that male fetal progenitor cells could persist postpartum in the maternal blood stream for as long as 27 years.[96]
A 2004 study at theFred Hutchinson Cancer Research Center,Seattle, investigated the origin of male chromosomes found in the peripheral blood of women who had not had male progeny. A total of 120 subjects (women who had never had sons) were investigated, and it was found that 21% of them had male DNA. The subjects were categorised into four groups based on their case histories:[97]
- Group A (8%) had had only female progeny.
- Patients in Group B (22%) had a history of one or more miscarriages.
- Patients Group C (57%) had their pregnancies medically terminated.
- Group D (10%) had never been pregnant before.
The study noted that 10% of the women had never been pregnant before, raising the question of where the Y chromosomes in their blood could have come from. The study suggests that possible reasons for occurrence of male chromosome microchimerism could be one of the following:[97]
- miscarriages,
- pregnancies,
- vanished male twin,
- possibly from sexual intercourse.
A 2012 study at the same institute has detected cells with the Y chromosome in multiple areas of the brains of deceased women.[98]
See also[edit]
- Genealogical DNA test
- Genetic genealogy
- Haplodiploid sex-determination system
- Human Y chromosome DNA haplogroups
- List of Y-STR markers
- Muller's ratchet
- Single nucleotide polymorphism
- Y chromosome Short Tandem Repeat (STR)
- Y linkage
- Y-chromosomal Aaron
- Y-chromosomal Adam
- Y-chromosome haplogroups in populations of the world
References[edit]
- ^ab"Homo sapiens Y chromosome genes".CCDS Release 20 for Homo sapiens.2016-09-08.Retrieved2017-05-28.
- ^Strachan T, Read A (2 April 2010).Human Molecular Genetics.Garland Science. p. 45.ISBN978-1-136-84407-2.
- ^abc"Ideogram data for Homo sapience (850 bphs, Assembly GRCh38.p3)".Genome Decoration Page.U.S. National Center for Biotechnology Information (NCBI). 2014-06-03.Retrieved2017-04-26.
- ^Glass B (1990)."Theophilus Shickel Painter: August 22, 1889-October 5, 1969"(PDF).Biographical Memoirs of the National Academy of Sciences.59:309–37.PMID11616163.
- ^abBainbridge D (2003).X in Sex: How the X Chromosome Controls.Cambridge, Mass.: Harvard University Press.ISBN978-0-674-01621-7.
- ^Schwartz J (2009).In Pursuit of the Gene: From Darwin to DNA.Cambridge, Mass.: Harvard University Press. pp. 170–2.ISBN978-0-674-03491-4.
- ^ab"Swyer syndrome — Symptoms, Causes, Treatment | NORD".rarediseases.org.Retrieved2023-09-12.
- ^abc"XX Male Syndrome — an overview".Science Direct.2023-09-12.
Dominguez AA, Reijo Pera RA (2013-01-01)."Infertility".In Maloy S, Hughes K (eds.).Brenner's Encyclopedia of Genetics(2nd ed.). Academic Press. pp. 71–74.doi:10.1016/B978-0-12-374984-0.00793-2.ISBN978-0-12-374984-0.Retrieved2023-09-12. - ^Devlin RH, Nagahama Y (2002-06-21)."Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences".Aquaculture.Sex determination and sex differentation in fish.208(3): 191–364.Bibcode:2002Aquac.208..191D.doi:10.1016/S0044-8486(02)00057-1.ISSN0044-8486.
- ^Barresi MJ, Gilbert SF (2023)."6. Sex Determination and Gametogenesis §6.4 Environmental Sex Determination in Reptiles".Developmental Biology(13th ed.). Oxford University Press.ISBN978-0-19-757459-1.
Gilbert SF (2000)."Environmental Sex Determination".Developmental Biology(6th ed.). Sinauer Associates. p. 60.ISBN0-87893-243-7.NBK9989. - ^Kuwamura T, Sunobe T, Sakai Y, Kadota T, Sawada K (2020-07-01)."Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system".Ichthyological Research.67(3): 341–360.Bibcode:2020IchtR..67..341K.doi:10.1007/s10228-020-00754-6.ISSN1616-3915.S2CID254168012.
- ^Muller HJ (1914)."A gene for the fourth chromosome of Drosophila".Journal of Experimental Zoology.17(3): 325–336.Bibcode:1914JEZ....17..325M.doi:10.1002/jez.1400170303.
- ^Lahn BT, Page DC (October 1999). "Four evolutionary strata on the human X chromosome".Science.286(5441): 964–7.doi:10.1126/science.286.5441.964.PMID10542153.
- ^abcdefghijkGraves JA (March 2006)."Sex chromosome specialization and degeneration in mammals".Cell.124(5): 901–914.doi:10.1016/j.cell.2006.02.024.PMID16530039.S2CID8379688.
- ^Graves JA, Koina E, Sankovic N (June 2006). "How the gene content of human sex chromosomes evolved".Current Opinion in Genetics & Development.16(3): 219–224.doi:10.1016/j.gde.2006.04.007.PMID16650758.
- ^abcBachtrog D (February 2013)."Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration".Nature Reviews. Genetics.14(2): 113–124.doi:10.1038/nrg3366.PMC4120474.PMID23329112.
- ^abWarren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grützner F, et al. (May 2008)."Genome analysis of the platypus reveals unique signatures of evolution".Nature.453(7192): 175–183.Bibcode:2008Natur.453..175W.doi:10.1038/nature06936.PMC2803040.PMID18464734.
- ^abcVeyrunes F, Waters PD, Miethke P, Rens W, McMillan D, Alsop AE, et al. (June 2008)."Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes".Genome Research.18(6): 965–973.doi:10.1101/gr.7101908.PMC2413164.PMID18463302.
- ^abGrützner F, Rens W, Tsend-Ayush E, El-Mogharbel N, O'Brien PC, Jones RC, et al. (December 2004). "In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes".Nature.432(7019): 913–7.Bibcode:2004Natur.432..913G.doi:10.1038/nature03021.PMID15502814.S2CID4379897.
- ^Watson JM, Riggs A, Graves JA (October 1992). "Gene mapping studies confirm the homology between the platypus X and echidna X1 chromosomes and identify a conserved ancestral monotreme X chromosome".Chromosoma.101(10): 596–601.doi:10.1007/BF00360536.PMID1424984.S2CID26978106.
- ^abLeMieux J (2020-05-29)."On PAR: How X and Y Chromosomes Recombine During Meiosis".GEN — Genetic Engineering and Biotechnology News.Retrieved2023-11-13.
- ^Peneder P, Wallner B, Vogl C (October 2017)."Exchange of genetic information between therian X and Y chromosome gametologs in old evolutionary strata".Ecology and Evolution.7(20): 8478–8487.Bibcode:2017EcoEv...7.8478P.doi:10.1002/ece3.3278.PMC5648654.PMID29075464.
- ^Dhanoa JK, Mukhopadhyay CS, Arora JS (July 2016)."Y-chromosomal genes affecting male fertility: A review".Vet World.9(7): 783–91.doi:10.14202/vetworld.2016.783-791.PMC4983133.PMID27536043.
- ^Brookfield JF (October 1995)."Human evolution. Y-chromosome clues to human ancestry".Current Biology.5(10): 1114–1115.Bibcode:1995CBio....5.1114B.doi:10.1016/S0960-9822(95)00224-7.PMID8548280.S2CID16081591.
- ^Graves JA (2004). "The degenerate Y chromosome--can conversion save it?".Reproduction, Fertility, and Development.16(5): 527–534.doi:10.1071/RD03096.PMID15367368.
- ^Goto H, Peng L, Makova KD (February 2009). "Evolution of X-degenerate Y chromosome genes in greater apes: conservation of gene content in human and gorilla, but not chimpanzee".Journal of Molecular Evolution.68(2): 134–144.Bibcode:2009JMolE..68..134G.doi:10.1007/s00239-008-9189-y.PMID19142680.S2CID24010421.
- ^Wade N (January 13, 2010)."Male Chromosome May Evolve Fastest".New York Times.
- ^Hughes JF, Skaletsky H, Pyntikova T, Minx PJ, Graves T, Rozen S, et al. (September 2005). "Conservation of Y-linked genes during human evolution revealed by comparative sequencing in chimpanzee".Nature.437(7055): 100–103.Bibcode:2005Natur.437..100H.doi:10.1038/nature04101.PMID16136134.S2CID4418662.
- ^Hsu C."Biologists Debunk the 'Rotting' Y Chromosome Theory, Men Will Still Exist".Medical Daily. Archived fromthe originalon 2012-02-25.Retrieved2012-02-23.
- ^Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. (December 2005)."Genome sequence, comparative analysis and haplotype structure of the domestic dog".Nature.438(7069): 803–819.Bibcode:2005Natur.438..803L.doi:10.1038/nature04338.PMID16341006.
- ^Brandeis M (May 2018). "New-age ideas about age-old sex: separating meiosis from mating could solve a century-old conundrum".Biological Reviews of the Cambridge Philosophical Society.93(2): 801–810.doi:10.1111/brv.12367.PMID28913952.S2CID4764175.
- ^Bernstein H, Hopf FA, Michod RE (1987). "The molecular basis of the evolution of sex".Molecular Genetics of Development.Advances in Genetics. Vol. 24. pp. 323–70.doi:10.1016/S0065-2660(08)60012-7.ISBN9780120176243.PMID3324702.
- ^Liu Z, Venkatesh SS, Maley CC (October 2008)."Sequence space coverage, entropy of genomes and the potential to detect non-human DNA in human samples".BMC Genomics.9(1): 509.doi:10.1186/1471-2164-9-509.PMC2628393.PMID18973670.Fig. 6, using theLempel-Zivestimators of entropy rate.
- ^Charlesworth B, Charlesworth D (November 2000)."The degeneration of Y chromosomes".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.355(1403): 1563–1572.doi:10.1098/rstb.2000.0717.PMC1692900.PMID11127901.
- ^abcRozen S, Skaletsky H, Marszalek JD, Minx PJ, Cordum HS, Waterston RH, et al. (June 2003). "Abundant gene conversion between arms of palindromes in human and ape Y chromosomes".Nature.423(6942): 873–876.Bibcode:2003Natur.423..873R.doi:10.1038/nature01723.PMID12815433.S2CID4323263.
- ^abHallast P, Balaresque P, Bowden GR, Ballereau S, Jobling MA (2013)."Recombination dynamics of a human Y-chromosomal palindrome: rapid GC-biased gene conversion, multi-kilobase conversion tracts, and rare inversions".PLOS Genet.9(7): e1003666.doi:10.1371/journal.pgen.1003666.PMC3723533.PMID23935520.
- ^Bonito M, D'Atanasio E, Ravasini F, Cariati S, Finocchio A, Novelletto A, Trombetta B, Cruciani F (November 2021)."New insights into the evolution of human Y chromosome palindromes through mutation and gene conversion".Hum Mol Genet.30(23): 2272–2285.doi:10.1093/hmg/ddab189.PMC8600007.PMID34244762.
- ^abMarchal JA, Acosta MJ, Bullejos M, Díaz de la Guardia R, Sánchez A (2003). "Sex chromosomes, sex determination, and sex-linked sequences in Microtidae".Cytogenetic and Genome Research.101(3–4): 266–273.doi:10.1159/000074347.PMID14684993.S2CID10526522.
- ^Wilson MA, Makova KD (2009). "Genomic analyses of sex chromosome evolution".Annual Review of Genomics and Human Genetics.10(1): 333–354.doi:10.1146/annurev-genom-082908-150105.PMID19630566.
- ^Just W, Baumstark A, Süss A, Graphodatsky A, Rens W, Schäfer N, et al. (2007). "Ellobius lutescens: sex determination and sex chromosome".Sexual Development.1(4): 211–221.doi:10.1159/000104771.PMID18391532.S2CID25939138.
- ^abcArakawa Y, Nishida-Umehara C, Matsuda Y, Sutou S, Suzuki H (2002). "X-chromosomal localization of mammalian Y-linked genes in two XO species of the Ryukyu spiny rat".Cytogenetic and Genome Research.99(1–4): 303–9.doi:10.1159/000071608.PMID12900579.S2CID39633026.
- ^Hoekstra HE, Edwards SV (September 2000)."Multiple origins of XY female mice (genus Akodon): phylogenetic and chromosomal evidence".Proceedings. Biological Sciences.267(1455): 1825–31.doi:10.1098/rspb.2000.1217.PMC1690748.PMID11052532.
- ^Ortiz MI, Pinna-Senn E, Dalmasso G, Lisanti JA (2009). "Chromosomal aspects and inheritance of the XY female condition inAkodon azarae(Rodentia, Sigmodontinae) ".Mammalian Biology.74(2): 125–9.Bibcode:2009MamBi..74..125O.doi:10.1016/j.mambio.2008.03.001.
- ^Charlesworth B, Dempsey ND (April 2001)."A model of the evolution of the unusual sex chromosome system of Microtus oregoni".Heredity.86(Pt 4): 387–394.doi:10.1046/j.1365-2540.2001.00803.x.PMID11520338.S2CID34489270.
- ^Zhou Q, Wang J, Huang L, Nie W, Wang J, Liu Y, et al. (2008)."Neo-sex chromosomes in the black muntjac recapitulate incipient evolution of mammalian sex chromosomes".Genome Biology.9(6): R98.doi:10.1186/gb-2008-9-6-r98.PMC2481430.PMID18554412.
- ^Hughes JF, Skaletsky H, Page DC (December 2012)."Sequencing of rhesus macaque Y chromosome clarifies origins and evolution of the DAZ (Deleted in AZoospermia) genes".BioEssays.34(12): 1035–44.doi:10.1002/bies.201200066.PMC3581811.PMID23055411.
- ^Hamilton WD (April 1967). "Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology".Science.156(3774): 477–488.Bibcode:1967Sci...156..477H.doi:10.1126/science.156.3774.477.PMID6021675.
- ^abSmith JJ, Voss SR (September 2007)."Bird and mammal sex-chromosome orthologs map to the same autosomal region in a salamander (ambystoma)".Genetics.177(1): 607–613.doi:10.1534/genetics.107.072033.PMC2013703.PMID17660573.
- ^Viana PF, Ezaz T, de Bello Cioffi M, Liehr T, Al-Rikabi A, Goll LG, et al. (July 2020)."Landscape of snake' sex chromosomes evolution spanning 85 MYR reveals ancestry of sequences despite distinct evolutionary trajectories".Scientific Reports.10(1): 12499.Bibcode:2020NatSR..1012499V.doi:10.1038/s41598-020-69349-5.PMC7385105.PMID32719365.
- ^Sahara K, Yoshido A, Traut W (January 2012)."Sex chromosome evolution in moths and butterflies".Chromosome Research.20(1): 83–94.doi:10.1007/s10577-011-9262-z.hdl:2115/49121.PMID22187366.S2CID15130561.
- ^Schartl M (July 2004). "A comparative view on sex determination in medaka".Mechanisms of Development.121(7–8): 639–645.doi:10.1016/j.mod.2004.03.001.PMID15210173.S2CID17401686.
- ^Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, et al. (April 2014). "Origins and functional evolution of Y chromosomes across mammals".Nature.508(7497): 488–493.Bibcode:2014Natur.508..488C.doi:10.1038/nature13151.PMID24759410.S2CID4462870.
- ^Deakin JE, Graves JA, Rens W (2012)."The evolution of marsupial and monotreme chromosomes".Cytogenetic and Genome Research.137(2–4): 113–129.doi:10.1159/000339433.hdl:1885/64794.PMID22777195.
- ^"Ensembl Human MapView release 43".February 2014.Retrieved2007-04-14.
- ^"National Library of Medicine's Genetic Home Reference".Archived fromthe originalon 2012-03-29.Retrieved2009-11-09.
- ^abcdefRhie A, Nurk S, Cechova M, Hoyt SJ, Taylor DJ, Altemose N, et al. (September 2023)."The complete sequence of a human Y chromosome".Nature.621(7978): 344–354.Bibcode:2023Natur.621..344R.doi:10.1038/s41586-023-06457-y.PMC10752217.PMID37612512.S2CID254181409.
- ^Pertea M, Salzberg SL (2010-05-05)."Between a chicken and a grape: estimating the number of human genes".Genome Biology.11(5): 206.doi:10.1186/gb-2010-11-5-206.PMC2898077.PMID20441615.
- ^"Definition of holandric".Dictionary.com.Retrieved2020-01-21.
- ^abHallast P, Ebert P, Loftus M, Yilmaz F, Audano PA, Logsdon GA, et al. (September 2023)."Assembly of 43 human Y chromosomes reveals extensive complexity and variation".Nature.621(7978): 355–364.Bibcode:2023Natur.621..355H.doi:10.1038/s41586-023-06425-6.PMC10726138.PMID37612510.S2CID261098546.
- ^"Ideogram data for Homo sapience (400 bphs, Assembly GRCh38.p3".Genome Decoration Page.U.S. National Center for Biotechnology Information (NCBI). 2014-06-04.Retrieved2017-04-26.
- ^"Ideogram data for Homo sapience (550 bphs, Assembly GRCh38.p3)".Genome Decoration Page.U.S. National Center for Biotechnology Information (NCBI). 2015-08-11.Retrieved2017-04-26.
- ^International Standing Committee on Human Cytogenetic Nomenclature (2013).ISCN 2013: An International System for Human Cytogenetic Nomenclature (2013).Karger Medical and Scientific Publishers.ISBN978-3-318-02253-7.
- ^Sethakulvichai W, Manitpornsut S, Wiboonrat M, Lilakiatsakun W, Assawamakin A, Tongsima S (2012). "Estimation of band level resolutions of human chromosome images".2012 Ninth International Conference on Computer Science and Software Engineering (JCSSE).pp. 276–282.doi:10.1109/JCSSE.2012.6261965.ISBN978-1-4673-1921-8.S2CID16666470.
- ^"p":Short arm;"q":Long arm.
- ^For cytogenetic banding nomenclature, see articlelocus.
- ^abThese values (ISCN start/stop) are based on the length of bands/ideograms from the ISCN book, An International System for Human Cytogenetic Nomenclature (2013).Arbitrary unit.
- ^gpos:Region which is positively stained byG banding,generallyAT-richand gene poor;gneg:Region which is negatively stained by G banding, generallyCG-richand gene rich;acenCentromere.var:Variable region;stalk:Stalk.
- ^"Scientists Reshape Y Chromosome Haplogroup Tree Gaining New Insights Into Human Ancestry".Science Daily.3 April 2008.
- ^Pertea M, Salzberg SL (2010)."Between a chicken and a grape: estimating the number of human genes".Genome Biology.11(5): 206.doi:10.1186/gb-2010-11-5-206.PMC2898077.PMID20441615.
- ^"Statistics & Downloads for chromosome Y".HUGO Gene Nomenclature Committee.2017-05-12. Archived fromthe originalon 2017-06-29.Retrieved2017-05-19.
- ^"Chromosome Y: Chromosome summary — Homo sapiens".Ensembl Release 88.2017-03-29.Retrieved2017-05-19.
- ^"Human chromosome Y: entries, gene names and cross-references to MIM".UniProt.2018-02-28.Retrieved2018-03-16.
- ^"Homo sapiens Y chromosome coding genes".National Center for Biotechnology Information Gene Database.2017-05-19.Retrieved2017-05-20.
- ^"Homo sapiens Y chromosome non-coding genes".2017-05-19.Retrieved2017-05-20.
- ^"Homo sapiens Y chromosome non-coding pseudo genes".2017-05-19.Retrieved2017-05-20.
- ^Colaco S, Modi D (February 2018)."Genetics of the human Y chromosome and its association with male infertility".Reproductive Biology and Endocrinology.16(1): 14.doi:10.1186/s12958-018-0330-5.PMC5816366.PMID29454353.
- ^Veerappa AM, Padakannaya P, Ramachandra NB (August 2013). "Copy number variation-based polymorphism in a new pseudoautosomal region 3 (PAR3) of a human X-chromosome-transposed region (XTR) in the Y chromosome".Functional & Integrative Genomics.13(3): 285–293.doi:10.1007/s10142-013-0323-6.PMID23708688.S2CID13443194.
- ^Raudsepp T, Chowdhary BP (6 January 2016)."The Eutherian Pseudoautosomal Region".Cytogenetic and Genome Research.147(2–3): 81–94.doi:10.1159/000443157.hdl:10576/22940.PMID26730606.
- ^Zeiher A, Braun T (July 2022). "Mosaic loss of Y chromosome during aging".Science.377(6603): 266–7.Bibcode:2022Sci...377..266Z.doi:10.1126/science.add0839.PMID35857599.S2CID250579530.
- ^abForsberg LA (May 2017)."Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men".Human Genetics.136(5): 657–663.doi:10.1007/s00439-017-1799-2.PMC5418310.PMID28424864.
- ^abForsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, Pakalapati G, et al. (June 2014)."Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer".Nature Genetics.46(6): 624–8.doi:10.1038/ng.2966.PMC5536222.PMID24777449.
- ^Guo X, Dai X, Zhou T, Wang H, Ni J, Xue J, Wang X (April 2020). "Mosaic loss of human Y chromosome: what, how and why".Human Genetics.139(4): 421–446.doi:10.1007/s00439-020-02114-w.PMID32020362.S2CID211036885.
- ^Sano S, Horitani K, Ogawa H, Halvardson J, Chavkin NW, Wang Y, et al. (July 2022)."Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality".Science.377(6603): 292–7.Bibcode:2022Sci...377..292S.doi:10.1126/science.abn3100.PMC9437978.PMID35857592.
- News article:Kolata G (14 July 2022)."As Y Chromosomes Vanish With Age, Heart Risks May Grow".The New York Times.Retrieved21 August2022.
- ^Coghlan A (13 December 2014)."Y men are more likely to get cancer than women".New Scientist:17.
- ^Dumanski JP, Rasi C, Lönn M, Davies H, Ingelsson M, Giedraitis V, et al. (January 2015)."Mutagenesis. Smoking is associated with mosaic loss of chromosome Y".Science.347(6217): 81–83.Bibcode:2015Sci...347...81D.doi:10.1126/science.1262092.PMC4356728.PMID25477213.
- ^"Loss of male sex chromosome leads to earlier death for men".University of Virginia.Retrieved31 August2022.
- ^"Loss of male sex chromosome may lead to earlier death for men – study".The Independent.14 July 2022.Retrieved31 August2022.
- ^abNussbaum, Robert L. (2007).Thompson & Thompson genetics in medicine.McInnes, Roderick R., Willard, Huntington F., Hamosh, Ada., Thompson, Margaret W. (Margaret Wilson), 1920- (7th. ed.). Philadelphia: Saunders/Elsevier.ISBN978-1416030805.OCLC72774424.
- ^Jacobs PA, Brunton M, Melville MM, Brittain RP, McClemont WF (December 1965). "Aggressive behavior, mental sub-normality and the XYY male".Nature.208(5017): 1351–2.Bibcode:1965Natur.208.1351J.doi:10.1038/2081351a0.PMID5870205.S2CID4145850.
- ^Richardson SS (2013).Sex Itself: The Search for Male & Female in the Human Genome.Chicago: U. of Chicago Press. p. 84.ISBN978-0-226-08468-8.
- ^Witkin HA, Mednick SA, Schulsinger F, Bakkestrom E, Christiansen KO, Goodenough DR, et al. (August 1976). "Criminality in XYY and XXY men".Science.193(4253): 547–555.Bibcode:1976Sci...193..547W.doi:10.1126/science.959813.PMID959813.
- ^Witkin HA, Goodenough DR, Hirschhorn K (October 1977). "XYY men: are they criminally aggressive?".The Sciences.17(6): 10–13.doi:10.1002/j.2326-1951.1977.tb01570.x.PMID11662398.
- ^Abedi M, Salmaninejad A, Sakhinia E (January 2018)."Rare 48, XYYY syndrome: case report and review of the literature".Clinical Case Reports.6(1): 179–184.doi:10.1002/ccr3.1311.PMC5771943.PMID29375860.
- ^Kopsida E, Stergiakouli E, Lynn PM, Wilkinson LS, Davies W (2009)."The Role of the Y Chromosome in Brain Function".Open Neuroendocrinology Journal.2:20–30.doi:10.2174/1876528900902010020.PMC2854822.PMID20396406.
- ^Schröder J, Tiilikainen A, De la Chapelle A (April 1974)."Fetal leukocytes in the maternal circulation after delivery. I. Cytological aspects".Transplantation.17(4): 346–354.doi:10.1097/00007890-197404000-00003.PMID4823382.S2CID35983351.
- ^Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA (January 1996)."Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum".Proceedings of the National Academy of Sciences of the United States of America.93(2): 705–8.Bibcode:1996PNAS...93..705B.doi:10.1073/pnas.93.2.705.PMC40117.PMID8570620.
- ^abYan Z, Lambert NC, Guthrie KA, Porter AJ, Loubiere LS, Madeleine MM, et al. (August 2005). "Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history".The American Journal of Medicine.118(8): 899–906.doi:10.1016/j.amjmed.2005.03.037.PMID16084184.
- ^Chan WF, Gurnot C, Montine TJ, Sonnen JA, Guthrie KA, Nelson JL (26 September 2012)."Male microchimerism in the human female brain".PLOS ONE.7(9): e45592.Bibcode:2012PLoSO...745592C.doi:10.1371/journal.pone.0045592.PMC3458919.PMID23049819.
External links[edit]
- CHM13v2.0 Y chromosome
- Ensembl genome browser
- Human Genome Project Information—Human Chromosome Y Launchpad
- On Topic: Y Chromosome—From the Whitehead Institute for Biomedical Research
- Nature—focus on the Y chromosome
- National Human Genome Research Institute (NHGRI)—Use of Novel Mechanism Preserves Y chromosome Genes
- Ysearch.org – Public Y-DNA databaseArchived2011-01-04 at theWayback Machine
- Y chromosome Consortium (YCC)Archived2017-01-16 at theWayback Machine
- NPR's Human Male: Still A Work In Progress
- Genetic Genealogy: About the use of mtDNA and Y chromosome analysis in ancestry testing