Yaws
| Yaws | |
|---|---|
| Other names | Frambesia tropica, thymosis, polypapilloma tropicum,[1]non-venereal endemic syphilis,[2]parangi and paru (Malay),[3]bouba (Spanish),[3]frambösie,[4]pian[5](French),[3]frambesia (German),[3]bakataw (Maguindanaoan)[3] |
 | |
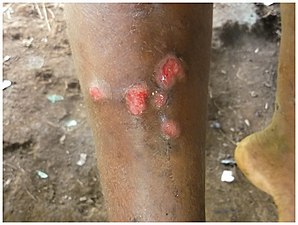
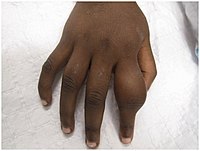
| Nodules on the elbow resulting from aTreponema pallidum pertenuebacterial infection | |
| Specialty | Infectious disease |
| Symptoms | Hard swelling of the skin,ulcer,joint and bone pain[6] |
| Causes | Treponema pallidumpertenuespread by direct contact |
| Diagnostic method | Based on symptoms, bloodantibodytests,polymerase chain reaction |
| Prevention | Mass treatment |
| Medication | Azithromycin,benzathine penicillin |
| Frequency | 46,000–500,000[7] |
Yawsis a tropicalinfectionof theskin,bones,and joints caused by thespirochetebacteriumTreponema pallidumpertenue.[6][7]The disease begins with a round, hard swelling of the skin, 2 to 5 cm (0.79 to 1.97 in) in diameter.[6]The center may break open and form anulcer.[6]This initial skin lesion typically heals after 3–6 months. After weeks to years, joints and bones may become painful,fatiguemay develop, and new skin lesions may appear.[6]The skin of thepalmsof the hands and the soles of the feet may become thick and break open. The bones (especially those of the nose) may become misshapen. After 5 years or more, large areas of skin may die, leaving scars.[6]
Yaws is spread by direct contact with the fluid from a lesion of an infected person. The contact is usually of a nonsexual nature. The disease is most common among children, who spread it by playing together.[6]Other relatedtreponemaldiseases arebejel(T. pallidum endemicum),pinta(T. carateum), andsyphilis(T. p. pallidum). Yaws is often diagnosed by the appearance of the lesions. Bloodantibodytests may be useful, but cannot separate previous from current infections.Polymerase chain reactionis the most accurate method of diagnosis.
No vaccine has yet been found.[8]Prevention is, in part, done by curing those who have the disease, thereby decreasing the risk of transmission. Where the disease is common, treating the entire community is effective. Improving cleanliness and sanitation also decreases spread. Treatment is typically withantibiotics,includingazithromycinby mouth orbenzathine penicillinby injection. Without treatment, physical deformities occur in 10% of cases.
Yaws is common in at least 13tropical countriesas of 2012.[6]Almost 85% of infections occurred in three countries—Ghana,Papua New Guinea,andSolomon Islands.[9]The disease only infects humans,[10]although 18th century French historianPierre François Xavier de Charlevoixdescribes it as a disease amonggeesein theAntilles.[11]Efforts in the 1950s and 1960s by theWorld Health Organizationdecreased the number of cases by 95%.[10]Since then, cases have increased, but with renewed efforts toglobally eradicatethe disease by 2020.[10]In 1995, the number of people infected was estimated at more than 500,000.[7]In 2016, the number of reported cases was 59,000.[12]Although one of the first descriptions of the disease was made in 1679 byWillem Piso,archaeological evidence suggests that yaws may have been present among human ancestors as far back as 1.6 million years ago.[6]
Signs and symptoms
[edit]Yaws primarily occurs in children, most frequently in those aged 6–10.[10]
Yaws is classified as primary, secondary, and tertiary; this can be clinically useful, but infected patients often have a mix of stages.[2]
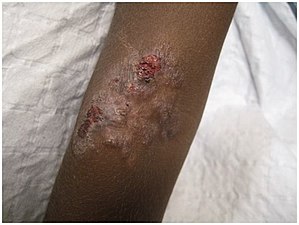
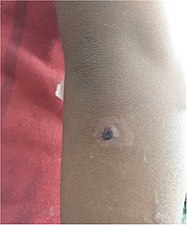
Within 9–90 days (but usually about 21 days[2]) of infection, a painless but distinctive "mother yaw"noduleappears.[2]Initiallyreddened and inflamed,[13]it may become apapilloma,which can then become anulcer,[10]possibly with a yellow crust.[14][better source needed]Mother yaws are most commonly found on the legs and ankles, and are rarely found on the genitals (unlike syphilis).[2]The mother yaw enlarges and becomes warty in appearance. Nearby "daughter yaws" may also appear simultaneously.[citation needed]This primary stage resolves completely, with scarring, within 3–6 months.[13]The scar is often pigmented.[2]
-
Papilloma mother yaw
-
Mother yaw nodule with central ulceration and a yellow crust
-
Ulcerated mother yaw
-
Ulcerated mother yaw
-
Healed primary yaw lesion, showing pigmented scar
The secondary stage occurs months to two years later (but usually 1–2 months later), and may thus begin when the mother yaw has not yet healed.[2]It happens when the bacterium spreads in the blood and lymph. It begins as multiple, pinhead-likepapules;these initial lesions grow and change in appearance and may last weeks before healing, with or without scarring.[2]
Secondary yaws typically shows widespread skin lesions that vary in appearance, including "crab yaws" (areas of skin of abnormal color) on the palms of the hands and soles of the feet[13](named for the crab-like gait they cause people with painful soles to assume[2]). These may showdesquamation.[citation needed]These secondary lesions frequently ulcerate and are then highly infectious, but heal after 6 months or more.[citation needed]
Secondary yaws affects the skin and bones.[13]The most common bone-related problem isperiostitis,an inflammation around the bone, often occurs in the bones of the fingers and the long bones of the lower arms and legs, causingswollen fingersand limbs.[13]This causes pain at night andthickening of the affected bones(periostitis).[2]About 75% of infected children surveyed in Papua New Guinea reported joint pain.[2]Swollen lymph nodes,fever, and malaise are also common.[13]
After primary and secondary yaws (and possibly, in some cases, without these phases), a latent infection develops.[2]Within five years (rarely, within ten years[2]) it can relapse and become active again, causing further secondary lesions, which may infect others.[13]These relapse lesions are most commonly found around the armpits, mouth, and anus.[2]
-
Secondary yaws begin as multiple small lesions.
-
The small lesions grow.
-
Secondary lesions vary in appearance (seelist of terms)
-
Here two different appearances (papulosquamous plaque and yellow-crustednodules) are seen in the same 10-year-old (large-scale of both, close-up of nodules)
-
Hypopigmentation and an crusted erosion, elbow of a 5-year-old
-
Secondary yaws;hypopigmentedareas of skin topped with pink and brownpapules,9-year-old
-
Erosion on the sole of the foot, close-up (large-scale). If deeper, it would be an ulcer
-
Secondary yaws papilloma (same 9-year-old as pictures of feet)
-
Secondary breakout in a 12-year-oldJavanesechild (wax model)
-
Secondary yaws scars in an adult with childhood history of yaws
An estimated 10% of people with yaws formerly were thought to develop tertiary disease symptoms, but more recently, tertiary yaws has been less frequently reported.[13][2]
Tertiary yaws can includegummatousnodules. It most commonly affects the skin. The skin of the palms and soles may thicken (hyperkeratosis). Nodules ulcerating near joints can causetissue death.Periostitis can be much more severe. The shinbones may become bowed (saber shin)[13]from chronic periostitis.[2]
Yaws may or may not havecardiovascularorneurologicaleffects; definitive evidence is lacking.[2]
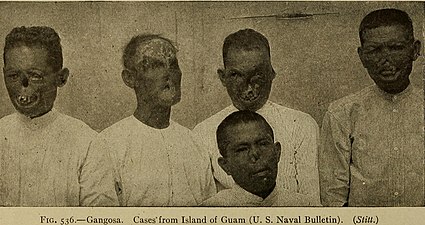
Rhinopharyngitis mutilans
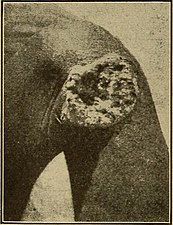
[edit]Rhinopharyngitis mutilans,[15][16]also known asgangosa,is a destructiveulcerativecondition that usually originates about thesoft palateand spreads into thehard palate,nasopharynx,andnose,resulting in mutilatingcicatrices,and outward to the face, eroding interveningbone,cartilage,andsoft tissues.It occurs in late stages of yaws, usually 5 to 10 years after first symptoms ofinfection.This is now rare.[2]Very rarely,[2]yaws may causebone spursin the upper jaw near the nose (gondou); gondou was rare even when yaws was a common disease.[13]
-
Deep ulceration occurs in tertiary yaws
-
Severe tertiary yaws; gangosa
-
Goundu, a very rare yaws-caused deformity around the nose
Cause
[edit]Yaws is caused by infection with bacteria of theTreponema pallidumsubspeciespertenue.[10]The initial yaws wound contains infectious bacteria, which are passed onto others through skin-to-skin contact, typically during play or other normal childhood interactions.[10][17]Early (primary and secondary) yaws lesions have a higher bacterial load, thus are more infectious.[2]Both papillomas and ulcers are infectious.[10]Infectivity is thought to last 12–18 months after infection, longer if a relapse occurs. Early yaws lesions are often itchy, and more lesions mayform along lines that are scratched.Yaws may be evolving less conspicuous lesions.[2]After a new person is infected, an infectious papilloma will form within 9–90 days (on average 21 days).[10]
T. pallidum pertenuehas been identified in nonhumanprimates(baboons,chimpanzees,andgorillas) and experimentalinoculationof human beings with asimianisolate causes yaws-like disease. However, no evidence exists of cross-transmission between human beings and primates, but more research is needed to discount the possibility of a yaws animal reservoir in nonhuman primates.[6]
Diagnosis
[edit]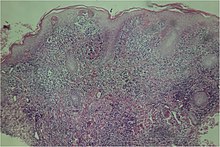
Most often the diagnosis is made clinically.[18]Dark field microscopyof samples taken from early lesions (particularly ulcerative lesions[18][verification needed]) may show the responsible bacteria; thespirochaetesare only 0.3 μm wide by 6–20 μm long, so light-field microscopy does not suffice.[2]
A microscopic examination of a biopsy of a yaw may show skin with clearepidermal hyperplasia(a type of skin thickening) andpapillomatosis(a type of surface irregularity), often with focalspongiosis(an accumulation of fluid in specific part of the epidermis).Immune systemcells,neutrophilsandplasma cells,accumulate in the skin, in densities that may cause microabscesses.[citation needed]
Warthin–Starryor Levaditi silver stains selectively stainT. pallidum,and direct and indirectimmunofluorescenceandimmunoperoxidasetests can detectpolyclonal antibodiestoT. pallidums. Histology often shows some spatial features which distinguish yaws from syphilis (syphilis is more likely to be found in the dermis, not the epidermis, and shows moreendothelial cellproliferation andvascularobliteration).[2]
Blood-serum (serological) testsare increasingly done at the point of care. They include a growing range oftreponemaland nontreponemal assays. Treponemal tests are morespecific,and are positive for any one who has ever been infected with yaws; they include theTreponema pallidumparticle agglutination assay.Nontreponemal assays can be used to indicate the progress of an infection and a cure, and positive results weaken and may become negative after recovery, especially after a case treated early.[13]They include the venereal disease research laboratory (VDRL;requires microscopy) and rapid plasma reagin (RPR;naked-eye result) tests, both of whichflocculatepatient-derivedantibodieswithantigens.[2]
Serological tests cannot distinguish yaws from the closely relatedsyphilis;[2]no test distinguishing yaws from syphilis is widely available. The two genomes differ by about 0.2%.PCRandDNA sequencingcan distinguish the two.[2]There are also no common blood tests which distinguish among the fourtreponematoses:syphilis (Treponema pallidum pallidum), yaws (Treponema pallidum pertenue), bejel (Treponema pallidum endemicum), and pinta (Treponema carateum).[18]
Haemophilus ducreyiinfections can cause skin conditions that mimic primary yaws. People infected withHaemophilus ducreyilesions may or may not also have latent yaws, and thus may or may not test positive on serological tests. This was discovered in the mid-2010s.[13]It seems that a recently diverged strain ofHaemophilus ducreyihas evolved from being a sexually transmitted infection to being a skin ulcer pathogen that looks like yaws.[19]
Yaws has been reported in nonendemic countries.[2]
Treatment
[edit]Treatment is normally by a singleintramuscular injectionof long-actingbenzathine benzylpenicillin,or less commonly by a course of other antibiotics, such asazithromycinortetracyclinetablets.[citation needed]Penicillin has been the front-line treatment since at least the 1960s, but there is no solid evidence of the evolution ofpenicillin resistancein yaws.[13]
The historical strategy for the eradication of yaws (1952–1964) was:[13]
| Prevalence of clinically active yaws | Treatment strategy |
|---|---|
| Hyperendemic: above 10% | Benzathine benzylpenicillin to the whole community
(total mass treatment) |
| Mesoendemic: 5–10% | Treat all active cases, all children under 15 and all contacts of infectious cases
(juvenile mass treatment) |
| Hypoendemic: under 5% | Treat all active cases and all household and other contacts
(selective mass treatment) |
Benzathine benzylpenicillin requires acold chainand staff who can inject it, and there is a small risk ofanaphylaxis.It was also not reliably available during the 2010s; there have been supply shortages.[13]
In the 2010s, a single oral dose ofazithromycinwas shown to be as effective as intramuscular penicillin.[20][13]Unlike penicillin, there is strong evidence that yaws is evolvingantibiotic resistanceto azithromycin; there are two known mutations in the bacterium, each of which can cause resistance and make the treatment ineffective. This has threatened eradication efforts.[13]
Within 8–10 hours of penicillin treatment, bacteria can no longer be found in lesion biopsies.[2]Primary and secondary lesions usually heal in 2–4 weeks; bone pain may improve within two days.[13]If treated early enough, bone deformities may reverse and heal.[2]Primary and secondary stage lesions may heal completely, but the destructive changes of tertiary yaws are largely irreversible.[citation needed]
If lesions do not heal, or RPR test results do not improve, this may indicate treatment failure or re-infection; the treatment is typically repeated.[2]WHO guidelines says that any presumed treatment failures at 4 weeks requiremacrolide resistancetesting.[10]
-
Secondary yaws in the left armpit of a ten-year-old, 2020
-
Same person, 2 weeks and 3.5 months after a single-dose azithromycin
-
Before and two weeks after a single injection of benzathine penicillin, 1950s.
Epidemiology
[edit]
Where the road ends, yaws begins
—WHO saying, quoted by Kingsley Asiedu.[22]
Yaws is typically found in humid tropical[13]forest regions inSouth America,Africa,AsiaandOceania.[8][10]
Yaws primarily affects children, with up to 80% of cases in those under 15 years of age, and peak incidence in children aged six to ten.[10]Boys and girls are impacted equally.[10]
It is more common in remote areas, where access to treatment is poorer.[13]It is associated with poverty and poor sanitation facilities and personal hygiene.[8][23][10]
Worldwide, almost 85% of yaws cases are in Ghana, Papua New Guinea, and the Solomon Islands. Rates in sub-Saharan Africa are low, but tend to be concentrated in specific populations. As of 2015[update],it is estimated that about 89 million people live in yaws-endemic areas, but data are poor, and this is likely an over-estimate.[23]
In the early 1900s, yaws was very common; in sub-saharan Africa, it was more frequently treated than malaria, sometimes making up more than half of treatments.[8]
Mass treatment campaigns in the 1950s reduced the worldwideprevalencefrom 50 to 150 million to fewer than 2.5 million; however, during the 1970s there were outbreaks inSouth-East Asia,and there have been continued sporadic cases in South America. As of 2011[update],it was unclear how many people worldwide were currently infected.[24]
From 2008 to 2012, 13 countries reported over 300,000 new cases to the WHO. There was no system for certifying local elimination of yaws, and it is not known whether the lack of reports from some countries is because they stopped having yaws cases or because they stopped reporting them. It is estimated that if there is not an active surveillance programme, there is less than a 1-in-2 chance that a country will successfully report yaws cases (if it gets them) in over three-quarters of countries with a history of yaws. These countries are thought to need international assistance to mount effective surveillance.[25]
History
[edit]Examination of remains ofHomo erectusfromKenya,that are about 1.6 million years old, has revealed signs typical of yaws. The genetic analysis of the yaws causative bacteria—Treponema pallidum pertenue—has led to the conclusion that yaws is the most ancient of the four knownTreponemadiseases. All otherTreponema pallidumsubspecies probably evolved fromTreponema pallidum pertenue.Yaws is believed to have originated in tropical areas of Africa, and spread to other tropical areas of the world via immigration and theslave trade.The latter is likely the way it was introduced toEuropefromAfricain the 15th century. The first unambiguous description of yaws was made by theDutchphysicianWillem Piso.Yaws was clearly described in 1679 amongAfrican slavesbyThomas Sydenhamin hisepistleonvenereal diseases,although he thought that it was the same disease assyphilis.The causative agent of yaws was discovered in 1905 byAldo Castellaniin ulcers of patients fromCeylon.[6]
The current English name is believed to be ofCariborigin, from "yaya", meaning sore.[18]
Towards the end of the Second World War yaws became widespread in the North of Malaya under Japanese occupation. After the country was liberated, the population was treated for yaws by injections ofsalvarsan,of which there was a great shortage, so only those with stage 1 were treated.[26]
Eradication
[edit]

A series of WHO yaws control efforts, which began shortly after creation of the WHO in 1948, succeeded in eradicating the disease locally from many countries, but have not lasted long enough to eradicate it globally. The Global Control of Treponematoses (TCP) programme by the WHO and theUNICEFwas launched in 1952 and continued until 1964. A 1953 questionnaire-based estimate was that there were 50–150 million yaws cases in 90 countries.[23]The global prevalence of yaws and the other endemic treponematoses,bejelandpinta,was reduced by the Global Control of Treponematoses (TCP) programme between 1952 and 1964 from about 50 million cases to about 2.5 million (a 95% reduction).[27]However, "premature integration of yaws and other endemic treponematoses activities into weak primary health-care systems, and the dismantling of the vertical eradication programmes after 1964, led to the failure to finish with the remaining 5% of cases"[27]and also led to a resurgence of yaws in the 1970s, with the largest number of case found in the Western Africa region.[24][28]Following the cessation of this program, resources, attention and commitment for yaws gradually disappeared and yaws remained at a low prevalence in parts of Asia, Africa, and the Americas with sporadic outbreaks. With few cases, mainly affecting poor, remote communities with little access to treatment, yaws became poorly known, yaws knowledge and skills died out even among health professionals, and yaws eradication was not seen as a high priority. Although a single injection of long-acting penicillin or otherbeta lactamantibiotic cures the disease and is widely available and the disease highly localised, many eradication campaigns ended in complacency and neglect; even in areas where transmission was successfully interrupted, re-introduction from infected areas occurred. Yaws eradication remained a priority in South-East Asia.[22][29]In 1995, the WHO estimated 460,000 worldwide cases.[30]
In the Philippines, yaws stopped being listed as anotifiable diseasein 1973; as of 2020, it is still present in the country.[3]
Indiaimplemented a successful Yaws eradication campaign that resulted in the 2016 certification by the WHO that India was free of yaws.[31][29][32]In 1996 there were 3,571 yaws cases in India; in 1997 after a serious elimination effort began the number of cases fell to 735. By 2003 the number of cases was 46. The last clinical case in India was reported in 2003 and the last latent case in 2006;[33]certification by the WHO was achieved in 2016.[31][34]
In 2012 the WHO officially targeted yaws for eradication by 2020 following the development of orally administered azithromycin as a treatment, but missed that target.[35][36][37]The Morges approach (named afterMorges,Switzerland, where a meeting on it was held[38]) involved mass treatment withazithromycin.This was safe, but ran into problems with antibiotic resistance, and did not fully interrupt transmission.[13]
The discovery that oral antibioticazithromycincan be used instead of the previous standard, injectedpenicillin,was tested onLihir Islandfrom 2013 to 2014;[39]a single oral dose of the macrolide antibiotic reduced disease prevalence from 2.4% to 0.3% at 12 months.[40]The WHO now recommends both treatment courses (oral azithromycin and injected penicillin), with oral azithromycin being the preferred treatment.[10]
As of 2020[update],there were 15 countries known to be endemic for yaws, with the recent discovery of endemic transmission inLiberiaand thePhilippines.[41]In 2020, 82,564 cases of yaws were reported to the WHO, and 153 cases were confirmed. The majority of the cases are reported fromPapua New Guineaand with over 80% of all cases coming from one of three countries in the 2010–2013 period: Papua New Guinea, Solomon Islands, and Ghana.[41][42]A WHO meeting report in 2018 estimated the total cost of elimination to be US$175 million (excluding Indonesia).[43]
In the South-East Asian Regional Office of the WHO, regional eradication efforts are focused on the remaining endemic countries in this region (IndonesiaandEast Timor)[44][45]afterIndiawas declared free of yaws in 2016.[46][43]
Although yaws is highly localized and eradication may be feasible, humans may not be the only reservoir of infection.[24]
References
[edit]- ^Maxfield L, Crane JS (January 2020). "Yaws (Frambesia tropica, Thymosis, Polypapilloma tropicum, Parangi, Bouba, Frambosie, Pian)".Stat Pearls.PMID30252269.
- ^abcdefghijklmnopqrstuvwxyzaaabacadaeMarks M, Lebari D, Solomon AW, Higgins SP (September 2015)."Yaws".International Journal of STD & AIDS.26(10): 696–703.doi:10.1177/0956462414549036.PMC4655361.PMID25193248.
- ^abcdefDofitas BL, Kalim SP, Toledo CB, Richardus JH (30 January 2020)."Yaws in the Philippines: first reported cases since the 1970s".Infectious Diseases of Poverty.9(1): 1.doi:10.1186/s40249-019-0617-6.PMC6990502.PMID31996251.
- ^Rapini RP, Bolognia JL, Jorizzo JL (2007).Dermatology: 2-Volume Set.St. Louis: Mosby.ISBN978-1-4160-2999-1.
- ^James WD, Berger TG, et al. (2006).Andrews' Diseases of the Skin: clinical Dermatology.Saunders Elsevier.ISBN0-7216-2921-0.OCLC62736861.
- ^abcdefghijkMitjà O, Asiedu K, Mabey D (2013). "Yaws".The Lancet.381(9868): 763–73.doi:10.1016/S0140-6736(12)62130-8.PMID23415015.S2CID208791874.
- ^abcMitjà O, Hays R, Rinaldi AC, McDermott R, Bassat Q (2012)."New treatment schemes for yaws: the path toward eradication"(pdf).Clinical Infectious Diseases.55(3): 406–412.doi:10.1093/cid/cis444.PMID22610931.Archivedfrom the original on 18 May 2014.
- ^abcdAsiedu K, Fitzpatrick C, Jannin J (25 September 2014)."Eradication of Yaws: Historical Efforts and Achieving WHO's 2020 Target".PLOS Neglected Tropical Diseases.8(9): 696–703.doi:10.1371/journal.pntd.0003016.ISSN1935-2727.PMC4177727.PMID25193248.
- ^Mitjà O, Marks M, Konan DJ, Ayelo G, Gonzalez-Beiras C, Boua B, Houinei W, Kobara Y, Tabah EN, Nsiire A, Obvala D, Taleo F, Djupuri R, Zaixing Z, Utzinger J, Vestergaard LS, Bassat Q, Asiedu K (June 2015)."Global epidemiology of yaws: a systematic review".The Lancet. Global Health.3(6): e324-31.doi:10.1016/S2214-109X(15)00011-X.PMC4696519.PMID26001576.
- ^abcdefghijklmno"Yaws".World Health Organization. 12 January 2023.Retrieved24 May2024.
- ^Charlevoix PF (1730).Histoire de l'isle Espagnole ou de S. Domingue.Paris: F. Barois. p. 29.
- ^"Number of cases of yaws reported".World Health Organization Global Health Observatory.Retrieved13 February2019.
- ^abcdefghijklmnopqrstuMarks M (29 August 2018)."Advances in the Treatment of Yaws".Tropical Medicine and Infectious Disease.3(3): 92.doi:10.3390/tropicalmed3030092.ISSN2414-6366.PMC6161241.PMID30274488.
- ^Yotsu RR (14 November 2018)."Integrated Management of Skin NTDs—Lessons Learned from Existing Practice and Field Research".Tropical Medicine and Infectious Disease.3(4): 120.doi:10.3390/tropicalmed3040120.ISSN2414-6366.PMC6306929.PMID30441754.
- ^L H B (1926). "Some observations on the tertiary lesions of framboesia tropica, or yaws".The American Journal of Tropical Medicine and Hygiene.1(2): 123–130.doi:10.4269/ajtmh.1926.s1-6.123.
- ^Berger S (1 February 2015).Infectious Diseases of Nauru.GIDEON Informatics Inc. p. 320.ISBN9781498805742.Retrieved31 October2015.
- ^Hotez 2022,p. 218.
- ^abcdDavis CP, Stoppler MC."Yaws".MedicineNet.com.Archivedfrom the original on 8 October 2012.Retrieved5 August2012.
- ^Lewis DA, Mitjà O (February 2016). "Haemophilus ducreyi: from sexually transmitted infection to skin ulcer pathogen".Current Opinion in Infectious Diseases.29(1): 52–57.doi:10.1097/QCO.0000000000000226.ISSN1473-6527.PMID26658654.S2CID1699547.
- ^Mitjà O, Hays, R, Ipai, A, Penias, M, Paru, R, Fagaho, D, de Lazzari, E, Bassat, Q (28 January 2012). "Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial".The Lancet.379(9813): 342–47.doi:10.1016/S0140-6736(11)61624-3.PMID22240407.S2CID17517869.
- ^"Countries where yaws is endemic".Our World in Data.Retrieved17 June2024.
- ^abAsiedu K (July 2008)."The return of yaws".Bulletin of the World Health Organization.86(7): 507–8.doi:10.2471/blt.08.040708.PMC2647480.PMID18670660.
- ^abcMitjà O, Marks M, Konan DJ, Ayelo G, Gonzalez-Beiras C, Boua B, Houinei W, Kobara Y, Tabah EN, Nsiire A, Obvala D, Taleo F, Djupuri R, Zaixing Z, Utzinger J, Vestergaard LS, Bassat Q, Asiedu K (19 May 2015)."Global epidemiology of yaws: a systematic review".The Lancet. Global Health.3(6): e324–e331.doi:10.1016/S2214-109X(15)00011-X.ISSN2214-109X.PMC4696519.PMID26001576.
- ^abcCapuano C, Ozaki M (2011)."Yaws in the Western Pacific Region: A Review of the Literature".Journal of Tropical Medicine.2011:642832.doi:10.1155/2011/642832.PMC3253475.PMID22235208.
- ^Fitzpatrick C, Asiedu K, Solomon AW, Mitja O, Marks M, Van der Stuyft P, Meheus F (4 December 2018)."Prioritizing surveillance activities for certification of yaws eradication based on a review and model of historical case reporting".PLOS Neglected Tropical Diseases.12(12): e0006953.doi:10.1371/journal.pntd.0006953.ISSN1935-2727.PMC6294396.PMID30513075.
- ^"All things uncertain: The story of the G.I.S." by Phyllis Stewart Brown
- ^ab"WHO renews efforts to achieve global eradication of yaws by 2020".
- ^Rinaldi A (2008)."Yaws: a second (and maybe last?) chance for eradication".PLOS Neglected Tropical Diseases.2(8): e275.doi:10.1371/journal.pntd.0000275.PMC2565700.PMID18846236.
- ^abAsiedu K, Amouzou B, Dhariwal A, Karam M, Lobo D, Patnaik S, Meheus A (2008)."Yaws eradication: past efforts and future perspectives".Bulletin of the World Health Organization.86(7): 499–500.doi:10.2471/BLT.08.055608.PMC2647478.PMID18670655.Archived fromthe originalon 21 April 2009.Retrieved2 April2009.
- ^"Integrating neglected tropical diseases in global health and development".Retrieved12 September2017.
- ^abFriedrich MJ (20 September 2016)."WHO Declares India Free of Yaws and Maternal and Neonatal Tetanus".JAMA.316(11): 1141.doi:10.1001/jama.2016.12649.ISSN1538-3598.PMID27654592.
- ^"World Health Organization South-East Asia | World Health Organization"(PDF).www.who.int.Archived fromthe originalon 8 November 2008.
- ^Akbar S (7 August 2011)."Another milestone for India: Yaws eradication".The Asian Age.Archivedfrom the original on 11 October 2011.Retrieved5 August2012.
- ^"Yaws Eradication Programme (YEP)".NCDC, Dte. General of Health Services, Ministry of Health & Family Welfare, Government of India.Archivedfrom the original on 2 February 2014.Retrieved18 January2014.
{{cite journal}}:Cite journal requires|journal=(help) - ^"Eradication of yaws – the Morges Strategy"(pdf).Weekly Epidemiological Record.87(20). 2012.Archived(PDF)from the original on 8 May 2014.Retrieved6 May2014.
- ^Maurice J (2012)."WHO plans new yaws eradication campaign".The Lancet.379(9824): 1377–78.doi:10.1016/S0140-6736(12)60581-9.PMID22509526.S2CID45958274.
- ^Rinaldi A (2012)."Yaws eradication: facing old problems, raising new hopes".PLOS Neglected Tropical Diseases.6(11): e18372.doi:10.1371/journal.pntd.0001837.PMC3510082.PMID23209846.
- ^"Summary report of a consultation on the eradication of yaws".WHO.Archived fromthe originalon 22 April 2017.
- ^Drug and a syphilis test offer hope of yaws eradicationArchived13 August 2013 at theWayback Machine,Thomas Reuter Foundation, accessed 10 May 2013
- ^Mitjà O, Houinei W, Moses P, Kapa A, Paru R, Hays R, Lukehart S, Godornes C, Bieb SV, Grice T, Siba P, Mabey D, Sanz S, Alonso PL, Asiedu K, Bassat Q (February 2015). "Mass treatment with single-dose azithromycin for yaws".The New England Journal of Medicine.372(8): 703–10.doi:10.1056/NEJMoa1408586.hdl:2445/68722.PMID25693010.S2CID5762563.
- ^ab"Yaws".www.who.int.
- ^Mitjà O, Marks M, Konan DJ, Ayelo G, Gonzalez-Beiras C, Boua B, Houinei W, Kobara Y, Tabah EN, Nsiire A, Obvala D, Taleo F, Djupuri R, Zaixing Z, Utzinger J, Vestergaard LS, Bassat Q, Asiedu K (19 May 2015)."Global epidemiology of yaws: a systematic review".The Lancet. Global Health.3(6): e324–e331.doi:10.1016/S2214-109X(15)00011-X.PMC4696519.PMID26001576.
- ^abOrganization WH (2018).Report of a global meeting on yaws eradication surveillance, monitoring and evaluation: Geneva, 29–30 January 2018.World Health Organization.hdl:10665/276314.
- ^Asiedu K, Amouzou B, Dhariwal A, Karam M, Lobo D, Patnaik S, Meheus A (July 2008)."Yaws eradication: past efforts and future perspectives".Bulletin of the World Health Organization.86(7): 499–499A.doi:10.2471/BLT.08.055608.PMC2647478.PMID18670655.
- ^Regional strategic plan for elimination of yaws from South-East Asia Region 2012-2020.WHO Regional Office for South-East Asia. 2013.hdl:10665/205830.
- ^Friedrich M (20 September 2016). "WHO Declares India Free of Yaws and Maternal and Neonatal Tetanus".JAMA.316(11): 1141.doi:10.1001/jama.2016.12649.PMID27654592.
Works cited
[edit]- Hotez PJ (2022). "12. The Newest NTDs and a Plea to" Repair the World "".Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development(3 ed.). John Wiley & Sons. pp. 217–226.ISBN9781683673897.
External links
[edit]- "Treponema pallidum subsp. pertenue".NCBI Taxonomy Browser.168.