Z-DNA
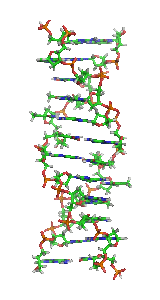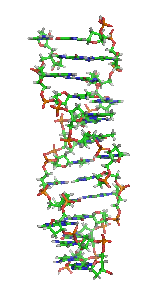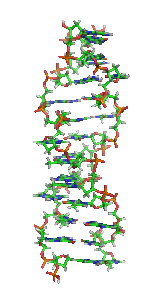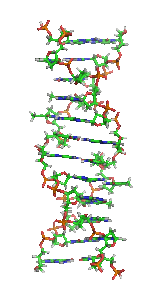
Z-DNAis one of the many possibledouble helicalstructures ofDNA.It is aleft-handeddouble helical structure in which the helix winds to the left in a zigzag pattern, instead of to the right, like the more commonB-DNAform. Z-DNA is thought to be one of three biologically active double-helical structures along withA-DNAandB-DNA.
History
[edit]Left-handed DNA was first proposed byRobert Wellsand colleagues, as the structure of a repeatingpolymerofinosine–cytosine.[1]They observed a "reverse"circular dichroismspectrum for such DNAs, and interpreted this incorrectly to mean that the strands wrapped around one another in a left-handed fashion. The relationship between Z-DNA and the more familiar B-DNA was indicated by the work of Pohl and Jovin,[2]who showed that theultravioletcircular dichroism of poly(dG-dC) was nearly inverted in4 Msodium chloridesolution and that the structure of poly d(I–C)·poly d(I–C) was in fact a right-handed D-DNA conformation. The suspicion that this was the result of a conversion from B-DNA to Z-DNA was confirmed by examining theRaman spectraof these solutions and the Z-DNA crystals.[3]Subsequently, acrystal structureof "Z-DNA" was published which turned out to be the first single-crystal X-ray structure of a DNA fragment (a self-complementary DNA hexamer d(CG)3). It was resolved as a left-handed double helix with twoantiparallelchains that were held together by Watson–Crickbase pairs(seeX-ray crystallography). It was solved byAndrew H. J. Wang,Alexander Rich,and coworkers in 1979 atMIT.[4]The crystallisation of a B- to Z-DNA junction in 2005[5]provided a better understanding of the potential role Z-DNA plays in cells. Whenever a segment of Z-DNA forms, there must be B–Z junctions at its two ends, interfacing it to the B-form of DNA found in the rest of thegenome.
In 2007, theRNAversion of Z-DNA,Z-RNA,was described as a transformed version of anA-RNAdouble helix into a left-handed helix.[6]The transition from A-RNA to Z-RNA, however, was already described in 1984.[7]
Structure
[edit]
Z-DNA is quite different from the right-handed forms. In fact, Z-DNA is often compared against B-DNA in order to illustrate the major differences. The Z-DNA helix is left-handed and has a structure that repeats every other base pair. The major and minor grooves, unlike A- and B-DNA, show little difference in width. Formation of this structure is generally unfavourable, although certain conditions can promote it; such as alternatingpurine–pyrimidinesequence (especially poly(dGC)2), negativeDNA supercoilingor high salt and somecations(all at physiological temperature, 37 °C, andpH7.3–7.4). Z-DNA can form a junction with B-DNA (called a "B-to-Z junction box" ) in a structure which involves the extrusion of a base pair.[8]The Z-DNA conformation has been difficult to study because it does not exist as a stable feature of the double helix. Instead, it is a transient structure that is occasionally induced by biological activity and then quickly disappears.[9]
Predicting Z-DNA structure
[edit]It is possible to predict the likelihood of a DNA sequence forming a Z-DNA structure. An algorithm for predicting the propensity of DNA to flip from the B-form to the Z-form,ZHunt,was written byP. Shing Hoin 1984 atMIT.[10]This algorithm was later developed byTracy Camp,P. Christoph Champ,Sandor Maurice,andJeffrey M. Vargasonfor genome-wide mapping of Z-DNA (with Ho as the principal investigator).[11]
Pathway of formation of Z-DNA from B-DNA
[edit]Since the discovery and crystallization of Z-DNA in 1979, the configuration has left scientists puzzled about the pathway and mechanism from the B-DNA configuration to the Z-DNA configuration.[12]The conformational change from B-DNA to the Z-DNA structure was unknown at the atomic level, but in 2010, computer simulations conducted by Lee et al. were able to computationally determine that the step-wise propagation of a B-to-Z transition would provide a lowerenergy barrierthan the previously hypothesized concerted mechanism.[13]Since this was computationally proven, the pathway would still need to be tested experimentally in the lab for further confirmation and validity, in which Lee et al. specifically states in their journal article, "The current [computational] result could be tested bySingle-molecule FRET(smFRET) experiments in the future. "[13]In 2018, the pathway from B-DNA to Z-DNA was experimentally proven using smFRET assays.[14]This was performed by measuring the intensity values between the donor and acceptor fluorescent dyes, also known asFluorophores,in relation to each other as they exchange electrons, while tagged onto a DNA molecule.[15][16]The distances between the fluorophores could be used to quantitatively calculate the changes in proximity of the dyes and conformational changes in the DNA. A Z-DNA high affinitybinding protein,hZαADAR1,[17]was used at varying concentrations to induce the transformation from B-DNA to Z-DNA.[14]The smFRET assays revealed a B* transition state, which formed as the binding of hZαADAR1 accumulated on the B-DNA structure and stabilized it.[14]This step occurs to avoid high junction energy, in which the B-DNA structure is allowed to undergo a conformational change to the Z-DNA structure without a major, disruptive change in energy. This result coincides with the computational results of Lee et al. proving the mechanism to be step-wise and its purpose being that it provides a lower energy barrier for the conformational change from the B-DNA to Z-DNA configuration.[13]Contrary to the previous notion, the binding proteins do not actually stabilize the Z-DNA conformation after it is formed, but instead they actually promote the formation of the Z-DNA directly from the B* conformation, which is formed by the B-DNA structure being bound by high affinity proteins.[14]
Biological significance
[edit]A biological role for Z-DNA in the regulation of type I interferon responses has been confirmed in studies of three well-characterized rare Mendelian Diseases: Dyschromatosis Symmetrica Hereditaria (OMIM: 127400), Aicardi-Goutières syndrome (OMIM: 615010) and Bilateral Striatal Necrosis/Dystonia. Families with haploid ADAR transcriptome enabled mapping of Zα variants directly to disease, showing that genetic information is encoded in DNA by both shape and sequence.[18]A role in regulating type I interferon responses in cancer is also supported by findings that 40% of a panel of tumors were dependent on the ADAR enzyme for survival.[19]
In previous studies, Z-DNA was linked to bothAlzheimer's diseaseandsystemic lupus erythematosus.To showcase this, a study was conducted on the DNA found in the hippocampus of brains that were normal, moderately affected with Alzheimer's disease, and severely affected with Alzheimer's disease. Through the use ofcircular dichroism,this study showed the presence of Z-DNA in the DNA of those severely affected.[20]In this study it was also found that major portions of the moderately affected DNA was in the B-Z intermediate conformation. This is significant because from these findings it was concluded that the transition from B-DNA to Z-DNA is dependent on the progression of Alzheimer's disease.[20]Additionally, Z-DNA is associated with systemic lupus erythematosus (SLE) through the presence of naturally occurring antibodies. Significant amounts of anti Z-DNA antibodies were found in SLE patients and were not present in other rheumatic diseases.[21]There are two types of these antibodies. Through radioimmunoassay, it was found that one interacts with the bases exposed on the surface of Z-DNA and denatured DNA, while the other exclusively interacts with the zig-zag backbone of only Z-DNA. Similar to that found in Alzheimer's disease, the antibodies vary depending on the stage of the disease, with maximal antibodies in the most active stages of SLE.
Z-DNA in transcription
[edit]Z-DNA is commonly believed to providetorsional strainrelief duringtranscription,and it is associated withnegative supercoiling.[5][22]However, while supercoiling is associated with both DNA transcription and replication, Z-DNA formation is primarily linked to the rate oftranscription.[23]
A study ofhuman chromosome 22showed a correlation between Z-DNA forming regions and promoter regions fornuclear factor I.This suggests that transcription in some human genes may be regulated by Z-DNA formation and nuclear factor I activation.[11]
Z-DNA sequences upstream of promoter regions have been shown to stimulate transcription. The greatest increase in activity is observed when the Z-DNA sequence is placed three helical turns after thepromoter sequence.Furthermore, using micrococcal nuclease-crosslinking technique,[24]Z-DNA is unlikely to formnucleosomes,which are often located before and/or after a Z-DNA forming sequence. Because of this property, Z-DNA is hypothesized to code for the boundary in nucleosome positioning. Since the placement of nucleosomes influences the binding oftranscription factors,Z-DNA is thought to regulate the rate of transcription.[24]
Developed behind the pathway ofRNA polymerasethrough negative supercoiling, Z-DNA formed via active transcription has been shown to increase genetic instability, creating a propensity towardsmutagenesisnear promoters.[25]A study onEscherichia colifound that genedeletionsspontaneously occur inplasmidregions containing Z-DNA-forming sequences.[26]In mammalian cells, the presence of such sequences was found to produce large genomic fragment deletions due to chromosomaldouble-strand breaks.Both of these genetic modifications have been linked to thegene translocationsfound in cancers such asleukemiaandlymphoma,since breakage regions intumor cellshave been plotted around Z-DNA-forming sequences.[25]However, the smaller deletions in bacterial plasmids have been associated withreplication slippage,while the larger deletions associated with mammalian cells are caused bynon-homologous end-joiningrepair, which is known to be prone to error.[25][26]
The toxic effect ofethidium bromide(EtBr) ontrypanosomasis caused by shift of theirkinetoplastidDNA to Z-form. The shift is caused byintercalationof EtBr and subsequent loosening of DNA structure that leads to unwinding of DNA, shift to Z-form and inhibition of DNA replication.[27]
Discovery of the Zα domain
[edit]The first domain to bind Z-DNA with high affinity was discovered inADAR1using an approach developed by Alan Herbert.[28][29]CrystallographicandNMRstudies confirmed the biochemical findings that this domain bound Z-DNA in a non-sequence-specific manner.[30][31][32]Related domains were identified in a number of other proteins throughsequence homology.[29]The identification of the Zα domain provided a tool for other crystallographic studies that lead to the characterization of Z-RNA and the B–Z junction. Biological studies suggested that the Z-DNA binding domain of ADAR1 may localize this enzyme that modifies the sequence of the newly formed RNA to sites of active transcription.[33][34]A role for Zα, Z-DNA and Z-RNA in defense of the genome against the invasion of Alu retro-elements in humans has evolved into a mechanism for the regulation of innate immune responses to dsRNA. Mutations in Zα are causal for human interferonopathies such as the Mendelian Aicardi-Goutières Syndrome.[35][18]Additionally, Zα domains are demonstrated to localize at the stress granules because of their innate ability in binding nucleic acid. Furthermore, different Zα domains bind to the Z conformation of nucleic acid differently providing important avenues for specific targeting in drug discovery.
Consequences of Z-DNA binding to vaccinia E3L protein
[edit]As Z-DNA has been researched more thoroughly, it has been discovered that the structure of Z-DNA can bind to Z-DNA binding proteins throughvan der Waal forcesandhydrogen bonding.[36]One example of a Z-DNA binding protein is thevacciniaE3L protein, which is a product of the E3L gene and mimics a mammalian protein that binds Z-DNA.[37][38]Not only does the E3L protein have affinity to Z-DNA, it has also been found to play a role in the level of severity of virulence in mice caused by vaccinia virus, a type ofpoxvirus.Two critical components to the E3L protein that determine virulence are theN-terminusand theC-terminus.The N-terminus is made of up a sequence similar to that of the Zα domain, also calledAdenosine deaminase z-alpha domain,while the C-terminus is composed of a double stranded RNA binding motif.[37]Through research done by Kim, Y. et al. at the Massachusetts Institute of Technology, it was shown that replacing the N-terminus of the E3L protein with a Zα domain sequence, containing 14 Z-DNA binding residues similar to E3L, had little to no effect on pathogenicity of the virus in mice.[37]In Contrast, Kim, Y. et al. also found that deleting all 83 residues of the E3L N-terminus resulted in decreased virulence. This supports their claim that the N-terminus containing the Z-DNA binding residues is necessary for virulence.[37]Overall, these findings show that the similar Z-DNA binding residues within the N-terminus of the E3L protein and the Zα domain are the most important structural factors determining virulence caused by the vaccinia virus, while amino acid residues not involved in Z-DNA binding have little to no effect. A future implication of these findings includes reducing Z-DNA binding of E3L in vaccines containing the vaccinia virus so negative reactions to the virus can be minimized in humans.[37]
Furthermore, Alexander Rich and Jin-Ah Kwon found that E3L acts as atransactivatorfor human IL-6, NF-AT, and p53 genes. Their results show thatHeLacells containing E3L had increased expression of human IL-6, NF-AT, and p53 genes and point mutations or deletions of certain Z-DNA binding amino acid residues decreased that expression.[36]Specifically, mutations in Tyr 48 and Pro 63 were found to reduce transactivation of the previously mentioned genes, as a result of loss of hydrogen bonding and london dispersion forces between E3L and the Z-DNA.[36]Overall, these results show that decreasing the bonds and interactions between Z-DNA and Z-DNA binding proteins decreases both virulence and gene expression, hence showing the importance of having bonds between Z-DNA and the E3L binding protein.
Comparison geometries of some DNA forms
[edit]
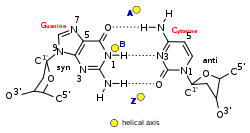
| A-form | B-form | Z-form | |
|---|---|---|---|
| Helix sense | right-handed | right-handed | left-handed |
| Repeating unit | 1 bp | 1 bp | 2 bp |
| Rotation/bp | 32.7° | 34.3° | 30° |
| bp/turn | 11 | 10 | 12 |
| Inclination of bp to axis | +19° | −1.2° | −9° |
| Rise/bp along axis | 2.3 Å (0.23 nm) | 3.32 Å (0.332 nm) | 3.8 Å (0.38 nm) |
| Pitch/turn of helix | 28.2 Å (2.82 nm) | 33.2 Å (3.32 nm) | 45.6 Å (4.56 nm) |
| Mean propeller twist | +18° | +16° | 0° |
| Glycosyl angle | anti | anti | C:anti, G:syn |
| Sugar pucker | C3′-endo | C2′-endo | C: C2′-endo, G: C3′-endo |
| Diameter | 23 Å (2.3 nm) | 20 Å (2.0 nm) | 18 Å (1.8 nm) |
See also
[edit]References
[edit]- ^Mitsui, Y.; Langridge, R.; Shortle, B. E.; Cantor, C. R.; Grant, R. C.; Kodama, M.; Wells, R. D. (1970). "Physical and enzymatic studies on poly d(I–C)·poly d(I–C), an unusual double-helical DNA".Nature.228(5277): 1166–1169.Bibcode:1970Natur.228.1166M.doi:10.1038/2281166a0.PMID4321098.S2CID4248932.
- ^Pohl, F. M.; Jovin, T. M. (1972). "Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly(dG-dC)".Journal of Molecular Biology.67(3): 375–396.doi:10.1016/0022-2836(72)90457-3.PMID5045303.
- ^Thamann, T. J.; Lord, R. C.; Wang, A. H.; Rich, A. (1981)."High salt form of poly(dG–dC)·poly(dG–dC) is left handed Z-DNA: raman spectra of crystals and solutions".Nucleic Acids Research.9(20): 5443–5457.doi:10.1093/nar/9.20.5443.PMC327531.PMID7301594.
- ^Wang, A. H.; Quigley, G. J.; Kolpak, F. J.; Crawford, J. L.; van Boom, J. H.; van der Marel, G.; Rich, A. (1979). "Molecular structure of a left-handed double helical DNA fragment at atomic resolution".Nature.282(5740): 680–686.Bibcode:1979Natur.282..680W.doi:10.1038/282680a0.PMID514347.S2CID4337955.
- ^abHa, S. C.; Lowenhaupt, K.; Rich, A.; Kim, Y. G.; Kim, K. K. (2005). "Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases".Nature.437(7062): 1183–1186.Bibcode:2005Natur.437.1183H.doi:10.1038/nature04088.PMID16237447.S2CID2539819.
- ^Placido, D.; Brown, B. A. II; Lowenhaupt, K.; Rich, A.; Athanasiadis, A. (2007)."A left-handed RNA double helix bound by the Zalpha domain of the RNA-editing enzyme ADAR1".Structure.15(4): 395–404.doi:10.1016/j.str.2007.03.001.PMC2082211.PMID17437712.
- ^Hall, K.; Cruz, P.; Tinoco, I. Jr; Jovin, T. M.; van de Sande, J. H. (Oct 1984). "'Z-RNA'—a left-handed RNA double helix ".Nature.311(5986): 584–586.Bibcode:1984Natur.311..584H.doi:10.1038/311584a0.PMID6482970.S2CID4316862.
- ^de Rosa, M.; de Sanctis, D.; Rosario, A. L.; Archer, M.; Rich, A.; Athanasiadis, A.; Carrondo, M. A. (May 2010)."Crystal structure of a junction between two Z-DNA helices".Proceedings of the National Academy of Sciences.107(20): 9088–9092.Bibcode:2010PNAS..107.9088D.doi:10.1073/pnas.1003182107.PMC2889044.PMID20439751.
- ^Zhang, H.; Yu, H.; Ren, J.; Qu, X. (2006)."Reversible B/Z-DNA transition under the low salt condition and non-B-form poly(dA)poly(dT) selectivity by a cubane-like europium-L-aspartic acid complex ".Biophysical Journal.90(9): 3203–3207.Bibcode:2006BpJ....90.3203Z.doi:10.1529/biophysj.105.078402.PMC1432110.PMID16473901.
- ^Ho, P. S.; Ellison, M. J.; Quigley, G. J.; Rich, A. (1986)."A computer aided thermodynamic approach for predicting the formation of Z-DNA in naturally occurring sequences".EMBO Journal.5(10): 2737–2744.doi:10.1002/j.1460-2075.1986.tb04558.x.PMC1167176.PMID3780676.
- ^abChamp, P. C.; Maurice, S.; Vargason, J. M.; Camp, T.; Ho, P. S. (2004)."Distributions of Z-DNA and nuclear factor I in human chromosome 22: a model for coupled transcriptional regulation".Nucleic Acids Research.32(22): 6501–6510.doi:10.1093/nar/gkh988.PMC545456.PMID15598822.
- ^Wang, Andrew H.-J.; Quigley, Gary J.; Kolpak, Francis J.; Crawford, James L.; van Boom, Jacques H.; van der Marel, Gijs; Rich, Alexander (December 1979). "Molecular structure of a left-handed double helical DNA fragment at atomic resolution".Nature.282(5740): 680–686.Bibcode:1979Natur.282..680W.doi:10.1038/282680a0.ISSN0028-0836.PMID514347.S2CID4337955.
- ^abcLee, Juyong; Kim, Yang-Gyun; Kim, Kyeong Kyu; Seok, Chaok (2010-08-05). "Transition between B-DNA and Z-DNA: Free Energy Landscape for the B−Z Junction Propagation".The Journal of Physical Chemistry B.114(30): 9872–9881.CiteSeerX10.1.1.610.1717.doi:10.1021/jp103419t.ISSN1520-6106.PMID20666528.
- ^abcdKim, Sook Ho; Lim, So-Hee; Lee, Ae-Ree; Kwon, Do Hoon; Song, Hyun Kyu; Lee, Joon-Hwa; Cho, Minhaeng; Johner, Albert; Lee, Nam-Kyung (2018-03-23)."Unveiling the pathway to Z-DNA in the protein-induced B–Z transition".Nucleic Acids Research.46(8): 4129–4137.doi:10.1093/nar/gky200.ISSN0305-1048.PMC5934635.PMID29584891.
- ^Cooper, David; Uhm, Heui; Tauzin, Lawrence J.; Poddar, Nitesh; Landes, Christy F. (2013-06-03)."Photobleaching Lifetimes of Cyanine Fluorophores Used for Single-Molecule Förster Resonance Energy Transfer in the Presence of Various Photoprotection Systems".ChemBioChem.14(9): 1075–1080.doi:10.1002/cbic.201300030.ISSN1439-4227.PMC3871170.PMID23733413.
- ^Didenko, Vladimir V. (November 2001)."DNA Probes Using Fluorescence Resonance Energy Transfer (FRET): Designs and Applications".BioTechniques.31(5): 1106–1121.doi:10.2144/01315rv02.ISSN0736-6205.PMC1941713.PMID11730017.
- ^Herbert, A.; Alfken, J.; Kim, Y.-G.; Mian, I. S.; Nishikura, K.; Rich, A. (1997-08-05)."A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase".Proceedings of the National Academy of Sciences.94(16): 8421–8426.Bibcode:1997PNAS...94.8421H.doi:10.1073/pnas.94.16.8421.ISSN0027-8424.PMC22942.PMID9237992.
- ^abHerbert, A. (2019)."Mendelian disease caused by variants affecting recognition of Z-DNA and Z-RNA by the Zα domain of the double-stranded RNA editing enzyme ADAR".European Journal of Human Genetics.8(1): 114–117.doi:10.1038/s41431-019-0458-6.PMC6906422.PMID31320745.
- ^Herbert, A. (2019). "ADAR and Immune Silencing in Cancer".Trends in Cancer.5(5): 272–282.doi:10.1016/j.trecan.2019.03.004.PMID31174840.S2CID155209484.
- ^abSuram, Anitha; Rao, Jagannatha K. S.; S., Latha K.; A., Viswamitra M. (2002). "First Evidence to Show the Topological Change of DNA from B-DNA to Z-DNA Conformation in the Hippocampus of Alzheimer's Brain".NeuroMolecular Medicine.2(3): 289–298.doi:10.1385/nmm:2:3:289.ISSN1535-1084.PMID12622407.S2CID29059186.
- ^Lafer, E M; Valle, R P; Möller, A; Nordheim, A; Schur, P H; Rich, A; Stollar, B D (1983-02-01)."Z-DNA-specific antibodies in human systemic lupus erythematosus".Journal of Clinical Investigation.71(2): 314–321.doi:10.1172/jci110771.ISSN0021-9738.PMC436869.PMID6822666.
- ^Rich, A; Zhang, S (2003). "Timeline: Z-DNA: the long road to biological function".Nature Reviews Genetics.4(7): 566–572.doi:10.1038/nrg1115.PMID12838348.S2CID835548.
- ^Wittig, B.; Dorbic, T.; Rich, A. (1991)."Transcription is associated with Z-DNA formation in metabolically active permeabilized mammalian cell nuclei".Proceedings of the National Academy of Sciences.88(6): 2259–2263.Bibcode:1991PNAS...88.2259W.doi:10.1073/pnas.88.6.2259.PMC51210.PMID2006166.
- ^abWong, B.; Chen, S.; Kwon, J.-A.; Rich, A. (2007)."Characterization of Z-DNA as a nucleosome-boundary element in yeastSaccharomyces cerevisiae".Proceedings of the National Academy of Sciences.104(7): 2229–2234.Bibcode:2007PNAS..104.2229W.doi:10.1073/pnas.0611447104.PMC1892989.PMID17284586.
- ^abcWang, G.; Christensen, L. A.; Vasquez, K. M. (2006)."Z-DNA-forming sequences generate large-scale deletions in mammalian cells".Proceedings of the National Academy of Sciences.108(8): 2677–2682.Bibcode:2006PNAS..103.2677W.doi:10.1073/pnas.0511084103.PMC1413824.PMID16473937.
- ^abFreund, A. M.; Bichara, M.; Fuchs, R. P. (1989)."Z-DNA-forming sequences are spontaneous deletion hot spots".Proceedings of the National Academy of Sciences.86(19): 7465–7469.Bibcode:1989PNAS...86.7465F.doi:10.1073/pnas.86.19.7465.PMC298085.PMID2552445.
- ^Roy Chowdhury, A.; Bakshi, R.; Wang, J.; Yıldırır, G.; Liu, B.; Pappas-Brown, V.; Tolun, G.; Griffith, J. D.; Shapiro, T. A.; Jensen, R. E.; Englund, P. T. (Dec 2010)."The killing of African trypanosomes by ethidium bromide".PLOS Pathogens.6(12): e1001226.doi:10.1371/journal.ppat.1001226.PMC3002999.PMID21187912.
- ^Herbert, A.; Rich, A. (1993)."A method to identify and characterize Z-DNA binding proteins using a linear oligodeoxynucleotide".Nucleic Acids Research.21(11): 2669–2672.doi:10.1093/nar/21.11.2669.PMC309597.PMID8332463.
- ^abHerbert, A.; Alfken, J.; Kim, Y. G.; Mian, I. S.; Nishikura, K.; Rich, A. (1997)."A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase".Proceedings of the National Academy of Sciences.94(16): 8421–8426.Bibcode:1997PNAS...94.8421H.doi:10.1073/pnas.94.16.8421.PMC22942.PMID9237992.
- ^Herbert, A.; Schade, M.; Lowenhaupt, K.; Alfken, J; Schwartz, T.; Shlyakhtenko, L. S.; Lyubchenko, Y. L.; Rich, A. (1998)."The Zα domain from human ADAR1 binds to the Z-DNA conformer of many different sequences".Nucleic Acids Research.26(15): 2669–2672.doi:10.1093/nar/26.15.3486.PMC147729.PMID9671809.
- ^Schwartz, T.; Rould, M. A.; Lowenhaupt, K.; Herbert, A.; Rich, A. (1999). "Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA".Science.284(5421): 1841–1845.doi:10.1126/science.284.5421.1841.PMID10364558.
- ^Schade, M.; Turner, C. J.; Kühne, R.; Schmieder, P.; Lowenhaupt, K.; Herbert, A.; Rich, A.; Oschkinat, H (1999)."The solution structure of the Zα domain of the human RNA editing enzyme ADAR1 reveals a prepositioned binding surface for Z-DNA".Proceedings of the National Academy of Sciences.96(22): 2465–2470.Bibcode:1999PNAS...9612465S.doi:10.1073/pnas.96.22.12465.PMC22950.PMID10535945.
- ^Herbert, A.; Rich, A. (2001)."The role of binding domains for dsRNA and Z-DNA in thein vivoediting of minimal substrates by ADAR1 ".Proceedings of the National Academy of Sciences.98(21): 12132–12137.Bibcode:2001PNAS...9812132H.doi:10.1073/pnas.211419898.PMC59780.PMID11593027.
- ^Halber, D. (1999-09-11)."Scientists observe biological activities of 'left-handed' DNA".MIT News Office.Retrieved2008-09-29.
- ^Herbert, A. (2019)."Z-DNA and Z-RNA in human disease".Communications Biology.2:7.doi:10.1038/s42003-018-0237-x.PMC6323056.PMID30729177.
- ^abcKwon, J.-A.; Rich, A. (2005-08-26)."Biological function of the vaccinia virus Z-DNA-binding protein E3L: Gene transactivation and antiapoptotic activity in HeLa cells".Proceedings of the National Academy of Sciences.102(36): 12759–12764.Bibcode:2005PNAS..10212759K.doi:10.1073/pnas.0506011102.ISSN0027-8424.PMC1200295.PMID16126896.
- ^abcdeKim, Y.-G.; Muralinath, M.; Brandt, T.; Pearcy, M.; Hauns, K.; Lowenhaupt, K.; Jacobs, B. L.; Rich, A. (2003-05-30)."A role for Z-DNA binding in vaccinia virus pathogenesis".Proceedings of the National Academy of Sciences.100(12): 6974–6979.Bibcode:2003PNAS..100.6974K.doi:10.1073/pnas.0431131100.ISSN0027-8424.PMC165815.PMID12777633.
- ^Kim, Y.-G.; Lowenhaupt, K.; Oh, D.-B.; Kim, K. K.; Rich, A. (2004-02-02)."Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection".Proceedings of the National Academy of Sciences.101(6): 1514–1518.Bibcode:2004PNAS..101.1514K.doi:10.1073/pnas.0308260100.ISSN0027-8424.PMC341766.PMID14757814.
- ^Sinden, Richard R. (1994).DNA Structure and Function(1st ed.). Academic Press. p. 398.ISBN978-0-126-45750-6.
- ^Rich, A.; Norheim, A.; Wang, A. H. (1984). "The chemistry and biology of left-handed Z-DNA".Annual Review of Biochemistry.53(1): 791–846.doi:10.1146/annurev.bi.53.070184.004043.PMID6383204.
- ^Ho, P. S. (1994-09-27)."The non-B-DNA structure of d(CA/TG)ndoes not differ from that of Z-DNA ".Proceedings of the National Academy of Sciences.91(20): 9549–9553.Bibcode:1994PNAS...91.9549H.doi:10.1073/pnas.91.20.9549.PMC44850.PMID7937803.
