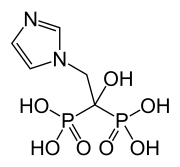
Zoledronic acid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Reclast, Zometa, others[1] |
| Other names | zoledronate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605023 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| Drug class | Bisphosphonate[3] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Protein binding | 22% |
| Metabolism | Nil |
| Eliminationhalf-life | 146 hours |
| Excretion | Kidney(partial) |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C5H10N2O7P2 |
| Molar mass | 272.090g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zoledronic acid,also known aszoledronateand sold under the brand nameZometaamong others,[7]byNovartisamong others, is amedicationused to treat a number ofbone diseases.[3]These includeosteoporosis,high blood calciumdue tocancer,bone breakdowndue to cancer,Paget's disease of bone[3]andDuchenne muscular dystrophy(DMD). It is given byinjection into a vein.[3]
Common side effects includefever,joint pain,high blood pressure,diarrhea, and feeling tired.[3]Serious side effects may includekidney problems,low blood calcium,andosteonecrosis of the jaw.[3]Use duringpregnancymay result in harm to the baby.[3]It is in thebisphosphonatefamily of medications.[3]It works by blocking the activity ofosteoclast cellsand thus decreases the breakdown of bone.[3]
Zoledronic acid was patented in 1986 and approved for medical use in the United States in 2001.[3][8]It is on theWorld Health Organization's List of Essential Medicines.[9]
Medical uses
[edit]Zoledronic acid isindicatedfor the prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumor-induced hypercalcemia) in people with advanced malignancies involving bone; the treatment of adults with tumor-induced hypercalcemia (TIH).[5]
Zoledronic acid is also indicated for the treatment and prevention of postmenopausal osteoporosis; the treatment to increase bone mass in men with osteoporosis; the treatment and prevention of glucocorticoid-induced osteoporosis; the treatment of Paget’s disease of bone in men and women.[4][6]
Bone complications of cancer
[edit]Zoledronic acid is used to preventbone fracturesin patients withcancerssuch asmultiple myelomaandprostate cancer,as well as for treatingosteoporosis.[10]It can also be used to treathypercalcaemiaof malignancy and can be helpful for treating pain frombone metastases.[11]
It can be given at home rather than in hospital. Such use has shown safety and quality-of-life benefits in people withbreast cancerand bone metastases.[12]
Osteoporosis
[edit]Zoledronic acid is used for the treatment ofosteoporosisin men and post-menopausal women at increased risk of fracture.[13][14]
In 2007, the USFood and Drug Administration(FDA) approved zoledronic acid for the treatment of postmenopausalosteoporosis.[7][15]
Other
[edit]Zoledronic acid may be used for treatment ofosteogenesis imperfecta.[16]
Contraindications
[edit]- Poorkidney function(e.g. estimated glomerular filtration rate less than 30 mL/min)[17]
- Hypocalcaemia
- Pregnancy
- Paralysis
Side effects
[edit]Side effects can includefatigue,anemia,muscle aches,fever,and/orswellingin the feet or legs.Flu-like symptomsare common after the first infusion, although not subsequent infusions, and are thought to occur because of its potential to activate humangamma delta T cell(γδ T cells).
Kidneys
[edit]There is a risk of severe renal impairment. Appropriate hydration is important before administration, as is adequatecalciumandvitamin Dintake before Aclasta therapy in patients withhypocalcaemia,and for ten days following Aclasta in patients with Paget's disease of the bone. Monitoring for other mineral metabolism disorders and the avoidance of invasive dental procedures for those who developosteonecrosis of the jawis recommended.[18]
Zoledronate is rapidly processed via thekidneys;consequently its administration is not recommended for patients with reducedrenal functionor kidney disease.[19]Some cases ofacute kidney injuryeither requiring dialysis or having a fatal outcome following Reclast use have been reported to the U.S.Food and Drug Administration(FDA).[20]This assessment was confirmed by theEuropean Medicines Agency(EMA), whose Committee for Medicinal Products for Human Use (CHMP) specified new contraindications for the medication on 15 December 2011, which include hypocalcaemia and severe renal impairment with acreatinineclearance of less than 35 ml/min.[21]
Bone
[edit]Osteonecrosis of the jaw
[edit]A rare complication that has been recently observed in cancer patients being treated with bisphosphonates isosteonecrosis of the jaw.This has mainly been seen in patients withmultiple myelomatreated with zoledronic acid who have haddental extractions.[22]
Atypical fractures
[edit]After approving the drug in July 2009, theEuropean Medicines Agencyconducted a class review of allbisphosphonates,including zoledronic acid, after several cases of atypical fractures were reported.[23]In 2008, the EMA's Pharmacovigilance Working Party (PhVWP) noted thatalendronic acidwas associated with an increased risk of atypical fracture of thefemurthat developed with low or no trauma. In April 2010, the PhVWP noted that further data from both the published literature and post-marketing reports were now available which suggested that atypical stress fractures of the femur may be a class effect. TheEuropean Medicines Agencythen reviewed all case reports of stress fractures in patients treated with bisphosphonates, relevant data from the published literature, and data provided by the companies which market bisphosphonates. The Agency recommended that doctors who prescribe bisphosphonate-containing medicines should be aware that atypical fractures may occur rarely in the femur, especially after long-term use, and that doctors who are prescribing these medicines for the prevention or treatment of osteoporosis should regularly review the need for continued treatment, especially after five or more years of use.[23]
Pharmacology
[edit]As anitrogenous bisphosphonate,zoledronic acid is a potent inhibitor ofbone resorption,allowing the bone-forming cells time to rebuild normalboneand allowingbone remodeling.[24] [25]
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
Research
[edit]Zoledronic acid has been found to have a direct antitumor effect and to synergistically augment the effects of other antitumor agents inosteosarcomacells.[27]
Zoledronic acid has shown significant benefits versus placebo over three years, with a reduced number ofvertebral fracturesand improved markers of bone density.[28][15]An annual dose of zoledronic acid may also prevent recurring fractures in patients with a previous hip fracture.[14]
With hormone therapy for breast cancer
[edit]An increase indisease-free survival(DFS) was found in the ABCSG-12 trial, in which 1,803 premenopausal women with endocrine-responsive early breast cancer receivedanastrozolewith zoledronic acid.[29]A retrospective analysis of the AZURE trial data revealed a DFS survival advantage, particularly where estrogen had been reduced.[30]
In a meta-analysis of trials where upfront zoledronic acid was given to preventaromatase inhibitor-associated bone loss, active cancer recurrence appeared to be reduced.[31]
As of 2010[update]"The results of clinical studies of adjuvant treatment on early-stage hormone-receptor-positive breast-cancer patients under hormonal treatment – especially with the bisphosphonate zoledronic acid – caused excitement because they demonstrated an additive effect on decreasing disease relapses at bone or other sites. A number of clinical andin vitroandin vivopreclinical studies, which are either ongoing or have just ended, are investigating the mechanism of action and antitumoral activity of bisphosphonates. "[32]
A 2010 review concluded that "adding zoledronic acid 4 mg intravenously every 6 months to endocrine therapy in premenopausal women with hormone receptor-positive early breast cancer... is cost-effective from a US health care system perspective".[33]
References
[edit]- ^"International trade names for zoledronic acid".Drugs.com.Archivedfrom the original on 4 March 2016.Retrieved14 January2015.
- ^"Zoledronic acid Use During Pregnancy".Drugs.com.1 June 2020.Archivedfrom the original on 16 November 2021.Retrieved19 October2020.
- ^abcdefghij"Zoledronic Acid".The American Society of Health-System Pharmacists.Archivedfrom the original on 15 December 2017.Retrieved8 December2017.
- ^ab"Reclast- zoledronic acid injection, solution".DailyMed.7 July 2022.Retrieved10 August2024.
- ^ab"Zometa EPAR".European Medicines Agency.20 March 2001.Archivedfrom the original on 7 June 2023.Retrieved5 July2024.Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ab"Aclasta EPAR".European Medicines Agency (EMA).15 April 2005.Retrieved10 August2024.Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ab"Novartis's Reclast Receives FDA Approval for Women With Postmenopausal Osteoporosis".FierceBiotech(Press release). 20 August 2007.Archivedfrom the original on 28 March 2018.Retrieved2 September2021.
- ^Fischer J, Ganellin CR (2006).Analogue-based Drug Discovery.John Wiley & Sons. p. 524.ISBN9783527607495.Archivedfrom the original on 14 January 2023.Retrieved2 June2020.
- ^World Health Organization(2023).The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023).Geneva: World Health Organization.hdl:10665/371090.WHO/MHP/HPS/EML/2023.02.
- ^National Prescribing Service (2009). "Zoledronic Acid for Osteoporosis".Medicines Update,Available at"Zoledronic acid (Aclasta) for osteoporosis: National Prescribing Service Ltd NPS".Archived fromthe originalon 23 April 2010.Retrieved20 January2010.
- ^"Zomera prescribing information"(PDF).Novartis Pharmaceuticals Corporation.U.S. Food and Drug Administration. April 2014.Archived(PDF)from the original on 19 June 2022.Retrieved10 October2023.
- ^Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, et al. (May 2005)."Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration".British Journal of Cancer.92(10): 1869–1876.doi:10.1038/sj.bjc.6602551.PMC2361764.PMID15870721.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Dhillon S (November 2016). "Zoledronic Acid (Reclast, Aclasta): A Review in Osteoporosis".Drugs.76(17): 1683–1697.doi:10.1007/s40265-016-0662-4.PMID27864686.S2CID22079489.
- ^abLyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. (November 2007)."Zoledronic acid and clinical fractures and mortality after hip fracture".The New England Journal of Medicine.357(18): 1799–1809.doi:10.1056/NEJMoa074941.PMC2324066.PMID17878149.
{{cite journal}}:CS1 maint: overridden setting (link) - ^abBlack DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. (May 2007)."Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis".The New England Journal of Medicine.356(18): 1809–1822.doi:10.1056/nejmoa067312.PMID17476007.S2CID71443125.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Dwan K, Phillipi CA, Steiner RD, Basel D (October 2016)."Bisphosphonate therapy for osteogenesis imperfecta".The Cochrane Database of Systematic Reviews.2016(10): CD005088.doi:10.1002/14651858.CD005088.pub4.PMC6611487.PMID27760454.
- ^Vondracek SF (April 2010). "Managing osteoporosis in postmenopausal women".American Journal of Health-System Pharmacy.67(7 Suppl 3): S9-19.doi:10.2146/ajhp100076.PMID20332498.
- ^"NPS MedicineWise"(PDF).Archived fromthe original(PDF)on 4 March 2016.Retrieved25 January2014.
- ^"Zometa 4mg/5ml Concentrate for Solution for Infusion".medicines.org.uk.Archived fromthe originalon 24 February 2010.Retrieved24 February2010.
- ^"FDA Alert: Reclast (zoledronic acid): Drug Safety Communication - New Contraindication and Updated Warning on Kidney Impairment".drugs.com.Archivedfrom the original on 3 March 2016.Retrieved23 January2018.
- ^"European Medicines Agency - Human medicines".europa.eu.Archived fromthe originalon 25 September 2015.Retrieved3 April2012.
- ^Durie BG, Katz M, Crowley J (July 2005)."Osteonecrosis of the jaw and bisphosphonates"(PDF).The New England Journal of Medicine.353(1): 99–102, discussion 99–102.doi:10.1056/NEJM200507073530120.PMID16000365.Archived(PDF)from the original on 21 August 2023.Retrieved25 June2023.
- ^ab"European Medicines Agency - Human medicines".europa.eu.Archived fromthe originalon 19 January 2013.Retrieved3 April2012.
- ^"Aclasta label- Australia"(PDF).Archived fromthe original(PDF)on 4 March 2016.Retrieved25 January2014.
- ^"Bisphosphonates".International Osteoporosis Foundation.Retrieved30 July2022.[permanent dead link]
- ^Tripathi KD (30 September 2013).Essentials of medical pharmacology(Seventh ed.). New Delhi: Jaypee Brothers Medical Publishers, Ltd.ISBN9789350259375.OCLC868299888.
- ^Koto K, Murata H, Kimura S, Horie N, Matsui T, Nishigaki Y, et al. (July 2010)."Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents".Oncology Reports.24(1): 233–239.doi:10.3892/or_00000851.PMID20514467.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. (February 2002)."Intravenous zoledronic acid in postmenopausal women with low bone mineral density".The New England Journal of Medicine.346(9): 653–661.doi:10.1056/NEJMoa011807.PMID11870242.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. (February 2009)."Endocrine therapy plus zoledronic acid in premenopausal breast cancer".The New England Journal of Medicine.360(7): 679–691.doi:10.1056/NEJMoa0806285.PMID19213681.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, et al. (March 2010)."The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer".British Journal of Cancer.102(7): 1099–1105.doi:10.1038/sj.bjc.6605604.PMC2853093.PMID20234364.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Brufsky A, Bundred N, Coleman R, Lambert-Falls R, Mena R, Hadji P, et al. (May 2008). "Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole".The Oncologist.13(5): 503–514.doi:10.1634/theoncologist.2007-0206.PMID18515735.S2CID23710758.
{{cite journal}}:CS1 maint: overridden setting (link) - ^Tonyali O, Arslan C, Altundag K (November 2010). "The role of zoledronic acid in the adjuvant treatment of breast cancer: current perspectives".Expert Opinion on Pharmacotherapy.11(16): 2715–2725.doi:10.1517/14656566.2010.523699.PMID20977404.S2CID26073229.
- ^Delea TE, Taneja C, Sofrygin O, Kaura S, Gnant M (August 2010). "Cost-effectiveness of zoledronic acid plus endocrine therapy in premenopausal women with hormone-responsive early breast cancer".Clinical Breast Cancer.10(4): 267–274.doi:10.3816/CBC.2010.n.034.PMID20705558.
