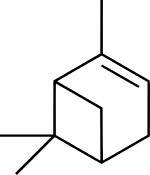
Pinene
| |
| Names | |
|---|---|
| IUPAC names
(1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
(1S,5S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.170 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Liquid |
| Density | 0,86 g·cm−3 (alpha, 15 °C)[1][2] |
| Melting point | −62 to −55 °C (−80 to −67 °F; 211 to 218 K) (alpha)[1] |
| Boiling point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) (alpha)[1] |
| Practically insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers.[3] Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca)[4] and big sagebrush (Artemisia tridentata).
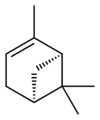
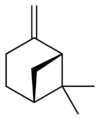
Isomers
[edit]| skeletal formula | 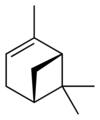 |
 |
 |

|
| perspective view | X | 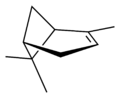 |
X | 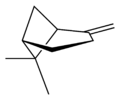
|
| ball-and-stick model | X | 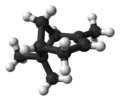 |
X | 
|
| name | (1R)-(+)-α-pinene | (1S)-(−)-α-pinene | (1R)-(+)-β-pinene | (1S)-(−)-β-pinene |
| CAS number | 7785-70-8 | 7785-26-4 | 19902-08-0 | 18172-67-3 |
Biosynthesis
[edit]α-Pinene and β-pinene are both produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate followed by loss of a proton from the carbocation equivalent. Researchers at the Georgia Institute of Technology and the Joint BioEnergy Institute have been able to synthetically produce pinene with a bacterium.[5]

Plants
[edit]Alpha-pinene is the most widely encountered terpenoid in nature[6] and is highly repellent to insects.[7]
Alpha-pinene appears in conifers and numerous other plants.[8] Pinene is a major component of the essential oils of Sideritis spp. (ironwort)[9] and Salvia spp. (sage).[10] Cannabis also contains alpha-pinene[8] and beta-pinene.[11] Resin from Pistacia terebinthus (commonly known as terebinth or turpentine tree) is rich in pinene. Pine nuts produced by pine trees contain pinene.[8]
Makrut lime fruit peel contains an essential oil comparable to lime fruit peel oil; its main components are limonene and β-pinene.[12]
The racemic mixture of the two forms of pinene is found in some oils like eucalyptus oil.[13]
Reactions
[edit]β-Pinene can be converted to α-pinene in the presence of strong bases.[14]
Selective oxidation of pinene occurs at the allylic position to give verbenone, along with pinene oxide, as well as verbenol and its hydroperoxide.[15][16]

Hydrogenation of pinene gives pinane, precursor to a useful pinanehydroperoxide.
The hydroboration of α-pinene has been extensively examined. With borane-dimethylsulfide, two equivalents of α-pinene react to give (diisopinocampheyl)borane.[17] Reaction with 9-BBN gives the reagent called alpine borane. This sterically crowded chiral trialkylborane can stereoselectively reduce aldehydes in what is known as the Midland Alpine borane reduction.[18]
Use
[edit]Pinenes, especially α, are the primary constituents of turpentine, a nature-derived solvent and fuel.[3]
The use of pinene as a biofuel in spark ignition engines has been explored.[19] Pinene dimers have been shown to have heating values comparable to the jet fuel JP-10.[5]
References
[edit]- ^ a b c Record of alpha-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 07-January-2016.
- ^ Record of beta-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 07-January-2016.
- ^ a b Gscheidmeier, Manfred; Fleig, Helmut (2000). "Turpentines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_267. ISBN 3527306730.
- ^ Lincoln DE, Lawrence BM (1984). "The Volatile Constituents of Camphorweed, Heterotheca subaxillaris". Phytochemistry. 23 (4): 933–934. Bibcode:1984PChem..23..933L. doi:10.1016/S0031-9422(00)85073-6.
- ^ a b Sarria S, Wong B, Martín HG, Keasling JD, Peralta-Yahya P (2014). "Microbial Synthesis of Pinene". ACS Synthetic Biology. 3 (7): 466–475. doi:10.1021/sb4001382. PMID 24679043.

- ^ Noma Y, Asakawa Y (2010). "Biotransformation of Monoterpenoids by Microorganisms, Insects, and Mammals". In Baser KH, Buchbauer G (eds.). Handbook of Essential Oils: Science, Technology, and Applications (2nd ed.). Boca Raton, FL: CRC Press. pp. 585–736. ISBN 9780429155666.
- ^ Nerio LS, Olivero-Verbel J, Stashenko E (2010). "Repellent activity of essential oils: a review". Bioresour Technol. 101 (1): 372–378. Bibcode:2010BiTec.101..372N. doi:10.1016/j.biortech.2009.07.048. PMID 19729299.
- ^ a b c Russo EB (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology. 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC 3165946. PMID 21749363.
- ^ Köse EO, Deniz İG, Sarıkürkçü C, Aktaş Ö, Yavuz M (2010). "Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr. (var. erythrantha and var. cedretorum P.H. Davis) endemic in Turkey". Food and Chemical Toxicology. 48 (10): 2960–2965. doi:10.1016/j.fct.2010.07.033. PMID 20670669.
- ^ Özek G, Demirci F, Özek T, Tabanca N, Wedge DE, Khan SI, et al. (2010). "Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity". Journal of Chromatography A. 1217 (5): 741–748. doi:10.1016/j.chroma.2009.11.086. PMID 20015509.
- ^ Hillig KW (2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis". Biochemical Systematics and Ecology. 32 (10): 875–891. Bibcode:2004BioSE..32..875H. doi:10.1016/j.bse.2004.04.004.
- ^ Kasuan N (2013). "Extraction of Citrus hystrix D.C. (Kaffir Lime) Essential Oil Using Automated Steam Distillation Process: Analysis of Volatile Compounds" (PDF). Malaysian Journal of Analytical Sciences. 17 (3): 359–369.
- ^ "alpha-Pinene - Compound Summary". PubChem. NCBI. Retrieved 14 Nov 2017.
- ^ Charles A. Brown, Prabhakav K. Jadhav (1987). "(a)-b-PINENE BY ISOMERIZATION OF (B)-b-PINENE". Organic Syntheses. 65: 224. doi:10.15227/orgsyn.065.0224.
- ^ Neuenschwander U, Guignard F, Hermans I (2010). "Mechanism of the Aerobic Oxidation of α-Pinene". ChemSusChem (in German). 3 (1): 75–84. Bibcode:2010ChSCh...3...75N. doi:10.1002/cssc.200900228. PMID 20017184.
- ^ Mark R. Sivik, Kenetha J. Stanton, Leo A. Paquette (1995). "(1R,5R)-(+)-Verbenone of High Optical Purity". Organic Syntheses. 72: 57. doi:10.15227/orgsyn.072.0057.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abbott, Jason; Allais, Christophe; Roush, William R. (2015). "Preparation of Crystalline (Diisopinocampheyl)borane". Organic Syntheses. 92: 26–37. doi:10.15227/orgsyn.092.0026.
- ^ M. Mark Midland "B-3-Pinanyl-9-borabicyclo[3.3.1]nonane" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley, New York.doi:10.1002/047084289X.rp173
- ^ Raman V, Sivasankaralingam V, Dibble R, Sarathy SM (2016). "α-Pinene - A High Energy Density Biofuel for SI Engine Applications". SAE Technical Paper. SAE Technical Paper Series. 1. doi:10.4271/2016-01-2171.
Bibliography
[edit]- Mann, J.; Davidson, R. S.; Hobbs, J. B.; Banthorpe, D. V.; Harborne, J. B. (1994). Natural Products. Harlow, UK: Addison Wesley Longman Ltd. pp. 309–311. ISBN 978-0-582-06009-8.
