Superplasticizer
Superplasticizers (SPs), also known as high range water reducers, are additives used for making high-strength concrete or to place self-compacting concrete. Plasticizers are chemical compounds enabling the production of concrete with approximately 15% less water content. Superplasticizers allow reduction in water content by 30% or more. These additives are employed at the level of a few weight percent. Plasticizers and superplasticizers also retard the setting and hardening of concrete.[1]
According to their dispersing functionality and action mode, one distinguishes two classes of superplasticizers:
- Ionic interactions (electrostatic repulsion): lignosulfonates (first generation of ancient water reducers), sulfonated synthetic polymers (naphthalene, or melamine, formaldehyde condensates) (second generation), and;
- Steric effects: Polycarboxylates-ether (PCE) synthetic polymers bearing lateral chains (third generation).[2]
Superplasticizers are used when well-dispersed cement particle suspensions are required to improve the flow characteristics (rheology) of concrete. Their addition allows to decrease the water-to-cement ratio of concrete or mortar without negatively affecting the workability of the mixture. It enables the production of self-consolidating concrete and high-performance concrete. The water–cement ratio is the main factor determining the concrete strength and its durability. Superplasticizers greatly improve the fluidity and the rheology of fresh concrete. The concrete strength increases when the water-to-cement ratio decreases because avoiding to add water in excess only for maintaining a better workability of fresh concrete results in a lower porosity of the hardened concrete, and so to a better resistance to compression.[3]
The addition of SP in the truck during transit is a fairly modern development within the industry. Admixtures added in transit through automated slump management system,[4] allow to maintain fresh concrete slump until discharge without reducing concrete quality.
Working mechanism
[edit]
Traditional plasticizers are lignosulphonates as their sodium salts.[5] Superplasticizers are synthetic polymers. Compounds used as superplasticizers include (1) sulfonated naphthalene formaldehyde condensate, sulfonated melamine formaldehyde condensate, acetone formaldehyde condensate and (2) polycarboxylates ethers. Cross-linked melamine- or naphthalene-sulfonates, referred to as PMS (polymelamine sulfonate) and PNS (polynaphthalene sulfonate), respectively, are illustrative. They are prepared by cross-linking of the sulfonated monomers using formaldehyde or by sulfonating the corresponding crosslinked polymer.[1][6]
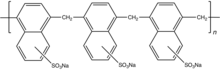
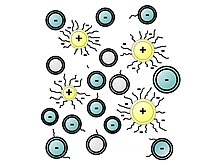
The polymers used as plasticizers exhibit surfactant properties. They are often ionomers bearing negatively charged groups (sulfonates, carboxylates, or phosphonates...). They function as dispersants to minimize particles segregation in fresh concrete (separation of the cement slurry and water from the coarse and fine aggregates such as gravels and sand respectively). The negatively charged polymer backbone adsorbs onto the positively charged colloidal particles of unreacted cement, especially onto the tricalcium aluminate (C3A) mineral phase of cement.
Melaminesulfonate (PMS) and naphthalenesulfonate (PNS) mainly act by electrostatic interactions with cement particles favoring their electrostatic repulsion while polycarboxylate-ether (PCE) superplasticizers sorb and coat large agglomerates of cement particles, and thanks to their lateral chains, sterically favor the dispersion of large cement agglomerates into smaller ones.[7]
However, as their working mechanisms are not fully understood, cement-superplasticizer incompatibilities can be observed in certain cases.[8]
Common superplasticizer types
[edit]- Sodium Lignosulfonate
- Sulfonated Naphthalene Formaldehyde
- Polycarboxylate Superplasticizer
Polycarboxylate superplasticizer (PCE), the third generation of high-performance superplasticizer,[9] follows the development of ordinary plasticizers and superplasticizers. It significantly reduces water content while enhancing concrete's workability, strength, and durability. Known for its cutting-edge technology, exceptional application prospects, and superior overall performance, PCE has revolutionized concrete admixtures.
See also
[edit]- Particle aggregation (inverse process of)
- Peptization
- Plasticizer
- Polycarboxylates
- Rheology
- Surfactant
- Suspension (chemistry)
References
[edit]- ^ a b Gerry Bye, Paul Livesey, Leslie Struble (2011). "Admixtures and Special Cements". Portland cement, Third edition. doi:10.1680/pc.36116.185 (inactive 2024-09-12). ISBN 978-0-7277-3611-6.
{{cite book}}: CS1 maint: DOI inactive as of September 2024 (link) CS1 maint: multiple names: authors list (link) - ^ Lu, Bing; Weng, Yiwei; Li, Mingyang; Qian, Ye; Leong, Kah Fai; Tan, Ming Jen; Qian, Shunzhi (May 2019). "A systematical review of 3D printable cementitious materials". Construction and Building Materials. 207: 477–490. doi:10.1016/j.conbuildmat.2019.02.144. hdl:10356/142503. S2CID 139995838.
- ^ Houst, Yves F.; Bowen, Paul; Perche, Francois; Kauppi, Annika; Borget, Pascal; Galmiche, Laurent; Le Meins, Jean-Francois; Lafuma, Francoise; Flatt, Robert J.; Schober, Irene; et al. (2008). "Design and function of novel superplasticizers for more durable high-performance concrete (Superplast project)". Cement and Concrete Research. 38 (10): 1197–1209. doi:10.1016/j.cemconres.2008.04.007.
- ^ "In-transit concrete management system | GCP Applied Technologies".
- ^ a b R. Flatt, I. Schober (2012). "Superplasticizers and the rheology of concrete". In Nicolas Roussel (ed.). Understanding the Rheology of Concrete. Woodhead. ISBN 978-0-85709-028-7.
- ^ Mollah, M. Y. A.; Adams, W. J.; Schennach, R.; Cocke, D. L. (2000). "A review of cement-superplasticizer interactions and their models". Advances in Cement Research. 12 (4): 153–161. doi:10.1680/adcr.2000.12.4.153.
- ^ Collepardi, M. (January 1998). "Admixtures used to enhance placing characteristics of concrete". Cement and Concrete Composites. 20 (2–3): 103–112. doi:10.1016/S0958-9465(98)00071-7. ISSN 0958-9465.
- ^ Ramachandran, V.S. (1995) Concrete Admixtures Handbook – Properties, Science, and Technology, 2nd Edition, William Andrew Publishing, ISBN 0-8155-1373-9 p. 121
- ^ polycarboxylate superplasticizer
Further reading
[edit]- Dodson, V.H. (29 June 2013). Concrete Admixtures. Springer Science & Business Media. ISBN 978-1-4757-4843-7.
External links
[edit]- Aïtcin, Pierre-Claude; Flatt, Robert J. (12 November 2015). Science and Technology of Concrete Admixtures. Woodhead Publishing. ISBN 978-0-08-100696-2.
