Rett syndrome
| Rett syndrome | |
|---|---|
| Other names | Cerebroatrophic hyperammonemia (obsolete),[1][2]dementia, ataxia, and loss of purposeful hand use syndrome[3] |
 | |
| A girl with Rett syndrome | |
| Specialty | Pediatric Neurology,Medical Genetics |
| Symptoms | Impairments in language and coordination, and repetitive movements, slower growth,smaller head[4] |
| Complications | Seizures,scoliosis,sleeping problems[4] |
| Usual onset | After 6–18 months of age[4] |
| Duration | Lifelong[5] |
| Causes | Mutation in theMECP2gene[4] |
| Diagnostic method | Based on symptoms,genetic testing[5] |
| Differential diagnosis | Angelman syndrome,autism,cerebral palsy,childhood disintegrative disorder,variousneurodegenerative disorders[6] |
| Treatment | Special education,physiotherapy, braces[5] |
| Medication | Anticonvulsants[5] |
| Prognosis | Life expectancy for many is middle age.[5] |
| Frequency | 1 in 8,500 females[4] Lethal in males, with rare exceptions. |
Rett syndrome(RTT) is agenetic disorderthat typically becomes apparent after 6-18 months of age and almost exclusively in girls.[4]Symptoms include impairments in language and coordination, and repetitive movements.[4]Those affected often have slower growth, difficulty walking, and asmaller head size.[4][5]Complications of Rett syndrome can includeseizures,scoliosis,andsleeping problems.[4]The severity of the condition is variable.[5]
Rett syndrome is due to a genetic mutation in theMECP2gene,[4]on theX chromosome.[5]It almost always occurs as a new mutation, with less than one percent of cases being inherited.[4][5]It occurs almost exclusively in girls;[4]boys who have a similar mutation typically die shortly after birth.[5]Diagnosis is based on the symptoms and can be confirmed withgenetic testing.[5]
There is no known cure for Rett syndrome.[5]Treatment is directed at improving symptoms.[5]Anticonvulsantsmay be used to help with seizures.[5]Special education,physiotherapy,and leg braces may also be useful depending on the needs of the child.[5]Many of those with the condition live into middle age.[5]
The condition affects about 1 in 8,500 females.[4]In 1999, Lebanese-American physicianHuda Zoghbidiscovered the mutation that causes the condition.[7][8]
Signs and symptoms
[edit]Stage I
[edit]Stage I, called early-onset, typically begins between 6 and 18 months of age.[5]This stage is often overlooked because symptoms of the disorder may be somewhat vague, and parents and doctors may not notice the subtle slowing of development at first.[5]The infant may begin to show less eye contact and have reduced interest in toys. There may be delays in gross motor skills such as sitting or crawling.[5]Hand-wringing and decreasing head growth may occur, but not enough to draw attention. This stage usually lasts for a few months but can continue for more than a year.[5]
Stage II
[edit]Stage II, or the rapid destructive stage, usually begins between ages 1 and 4 and may last for weeks or months.[5]Its onset may be rapid or gradual as the child loses purposeful hand skills and spoken language.[5]Characteristic hand movements such as wringing, washing, clapping, or tapping, as well as repeatedly moving the hands to the mouth often begin during this stage which is called mouthing.[5]The child may hold the hands clasped behind the back or held at the sides, with random touching, grasping, and releasing.[5]The movements continue while the child is awake but disappear during sleep.[5]Breathing irregularities such as episodes of apnea and hyperventilation may occur, although breathing usually improves during sleep.[5]Some girls also display autistic-like symptoms such as loss of social interaction and communication.[5]Walking may be unsteady and initiating motor movements can be difficult. Slowed head growth is usually noticed during this stage.[5]
Stage III
[edit]Stage III, or the plateau or pseudo-stationary stage, usually begins between ages 2 and 10 and can last for years.[5]Apraxia,motor problems, andseizuresare prominent during this stage.[5]However, there may be improvement in behavior, with less irritability, crying, andautistic-like features.[5]In stage III there may be more interest in the surroundings and alertness, attention span, and communication skills may improve.[5]Many girls remain in this stage for most of their lives.[5]
Stage IV
[edit]Stage IV, or the late motor deterioration stage, can last for years or decades.[5]Prominent features include reduced mobility,curvature of the spine,and muscle weakness, rigidity, spasticity, and increased muscle tone with abnormal posturing of an arm or leg.[5]Girls who were previously able to walk may stop walking.[5]Cognition, communication, or hand skills generally do not decline in stage IV.[5]Repetitive hand movements may decrease and eye gaze usually improves.[5]
Variants
[edit]The signs and symptoms of the typical form of the Rett syndrome are well described. In addition to the classical form of Rett syndrome, several atypical forms have been described over the years;[9]the main groups are:
- Congenital variant (Rolando variant): in this severe subtype of Rett syndrome, the development of the patients and their head circumference are abnormal from birth.[10]The typical gaze of Rett syndrome patients is usually absent;
- Zappellavariant of Rett Syndrome or preserved speech variant: in this subtype of Rett syndrome the patients acquire some manual skills and language is partially recovered around the age of 5 years (that is after the regression phase). Height, weight and head circumference are often in the normal range, and a good gross motor function can be observed.[11][12][13][14][15][16]The Zappella variant is a milder form of Rett syndrome;
- Hanefeld variant or early epilepsy variant. In this form of Rett syndrome, the patients have epilepsy before 5 months of age.[17]
The definition itself of the Rett syndrome has been refined over the years: as the atypical forms subsist near to the classical form (Hagberg & Gillberg, 1993), the "Rett Complex" terminology has been introduced.[18][19]
Cause
[edit]Genetically, Rett syndrome (RTT) is often caused by mutations in the geneMECP2[20]located on the X chromosome (which is involved in transcriptional silencing and epigenetic regulation of methylated DNA), and can arise sporadically or from germline mutations. In less than 10% of RTT cases, mutations in the genesCDKL5orFOXG1have also been found to resemble it.[21][22]Rett syndrome is initially diagnosed by clinical observation, and is commonly associated with a genetic defect in the MECP2 gene.[20]
It has been argued that Rett syndrome is in fact a neurodevelopmental condition as opposed to a neurodegenerative condition. One piece of evidence for this is that mice with induced Rett Syndrome show no neuronal death, and some studies have suggested that their phenotypes can be partially rescued by adding functional MECP2 gene back when they are adults. This information has also helped lead to further studies aiming to treat the disorder.[23]
Sporadic mutations
[edit]In at least 95% of Rett syndrome cases, the cause is ade novomutationin the child, almost exclusively from a de novo mutation on the male copy of the X chromosome.[24][25]It is not yet known what causes the sperm to mutate, and such mutations are rare.
Germline mutations
[edit]It can also be inherited from phenotypically normal mothers who have agermlinemutation in the gene encodingmethyl-CpG-binding protein-2,MeCP2.[26]In these cases, inheritance follows anX-linked dominantpattern and is seen almost exclusively in females, as most males diein uteroor shortly after birth.[27]MECP2 is found near the end of the long arm of the X chromosome at Xq28. An atypical form of RTT, characterized by infantile spasms or early onset epilepsy, can also be caused by a mutation to the gene encodingcyclin-dependent kinase-like 5(CDKL5). As stated by Aine Merwick, Margaret O'Brien, and Norman Delanty in an article on gene disorders titledComplex single gene disorders and epilepsy,"Rett syndrome affects one in every 12,500 female live births by age 12 years."[28]
Mechanism
[edit]Pontine noradrenergic deficits
[edit]Brain levels ofnorepinephrineare lower in people with Rett syndrome[29](reviewed in[30]). The genetic loss ofMECP2changes the properties of cells in thelocus coeruleus,the exclusive source of noradrenergic innervation to thecerebral cortexandhippocampus.[31][32]These changes include hyperexcitability and decreased functioning of its noradrenergic innervation.[33]Moreover, a reduction of thetyrosine hydroxylase(Th) mRNA level, the rate-limiting enzyme in catecholamine synthesis, was detected in the wholeponsofMECP2-null male as well as in adult heterozygous (MECP2+/-) female mice.[34]Using immunoquantitative techniques, a decrease of Th protein staining level, number of locus coeruleus Th-expressing neurons and density ofdendritic arborizationsurrounding the structure was shown in symptomaticMeCP2-deficient mice.[34]However, locus coeruleus cells are not dying, but are more likely losing their fully mature phenotype, since no apoptotic neurons in the pons were detected.[34]
Researchers have concluded that "Because these neurons are a pivotal source of norepinephrine throughout the brainstem and forebrain and are involved in the regulation of diverse functions disrupted in Rett syndrome, such as respiration and cognition, we hypothesize that the locus coeruleus is a critical site at which loss ofMECP2results in CNS dysfunction. "The restoration of normal locus coeruleus function may therefore be of potential therapeutic value in the treatment of Rett syndrome.[33][34]
Midbrain dopaminergic disturbances
[edit]The majority ofdopaminein the mammalian brain is synthesized by nuclei located in themesencephalon.Thesubstantia nigrapars compacta (SNpc), theventral tegmental area(VTA) and theretrorubral field(RRF) contain dopaminergic neurons expressing tyrosine hydroxylase (Th, i.e. the rate-limiting enzyme in catecholamine synthesis).[35][36][37]
The nigro-striatal pathway originates from the SNpc; its principal rostral target is the caudate-putamen (CPu), which it irradiates through the median forebrain bundle (MFB). This connection is involved in the tight modulation of motor strategies computed by a cortico-basal ganglia-thalamo-cortical loop.[38]
Indeed, based on the canonical anatomofunctional model of basal ganglia, nigrostriatal dopamine is able to modulate the motor loop by acting on dopaminergic receptors located on striatal GABAergic medium spiny neurons.[39]
Dysregulation of the nigrostriatal pathway is causative from Parkinson disease (PD) in humans.[40]Toxic and/or genetic ablation of SNpc neurons produces experimental parkinsonism in mice and primates.[41]The common features of PD and PD animal models are motor impairments[42](hypotonia, bradykinesia, hypokinesia).
RTT pathology, in some aspects, overlaps the motor phenotype observed in PD patients.[43][44][45]Several neuropathological studies on postmortem brain samples argued for an SNpc alteration, evidenced by neuromelanin hypopigmentation, reduction in the structure area, and even, controversially, signs of apoptosis. In parallel, a hypometabolism was underlined by a reduction of several catecholamines (dopamine, noradrenaline, adrenaline) and their principal metabolic by-products.[30]Mouse models of RTT are available; the most studied are constitutively deletedMecp2mice developed byAdrian BirdorKatelyn McCormicklaboratories.[46][47][48][49]
In accordance with the motor spectrum of the RTT phenotype,Mecp2-null mice show motor abnormalities from postnatal day 30 that worsen until death. These models offer a crucial substrate to elucidate the molecular and neuroanatomical correlates ofMeCP2-deficiency.[50]Recently (2008), it was shown that the conditional deletion ofMecp2in catecholaminergic neurons (by crossing of Th-Cre mice with loxP-flankedMecp2ones) recapitulates a motor symptomatology; it was further documented that brain levels of Th in mice lackingMeCP2in catecholaminergic neurons only are reduced, participating to the motor phenotype.[51]
However, the most studied model for the evaluation of therapeutics is theMecp2-null mouse(totally devoid ofMeCP2). In this context, a reduction in the number and soma size of Th-expressing neurons is present from 5 weeks of age and is accompanied by a decrease of Th immunoreactivity in the caudate-putamen, the principal target of dopaminergic neurons arising from the SNpc.[52]Moreover, a neurochemical analysis of dopaminergic contents in microdissected midbrain and striatal areas revealed a reduction of dopamine at five and nine weeks of age. It is noteworthy that later on (at nine weeks), the morphological parameters remain altered but not worsened, whereas the phenotype progresses and behavioral deficits are more severe. The amount of fully activated Th (Serine40-phosphorylated isoform) in neurons that remain in the SNpc is mildly affected at 5 weeks but severely impaired by 9 weeks.[52]Finally, using a chronic and oral L-Dopa treatment onMeCP2-deficient mice, authors reported an amelioration of some of the motor deficits previously identified.[52]Altogether, these results argue for an alteration of the nigrostriatal dopaminergic pathway inMeCP2-deficient animals as a contributor of the neuromotor deficits.[52]
There is an association of Rett syndrome withbrain-derived neurotrophic factor(BDNF).[53]
Molecular functions of MECP2 in Rett syndrome pathology
[edit]As reviewed by Sharifi and Yasui,[54]MECP2protein, encoded by theMECP2gene binds to DNA with a high affinity forCpG methylated DNA sitesand affectstranscription.MECP2 can bind to 5mc (5-methylcytosine) and 5hmc (5-hydroxymethylcytosine) with similar affinity, and these dinucleotides account for the majority of MECP2 binding sites in the mammaliangenome.MECP2 is involved in higher orderchromatinorganization and appears necessary for compacting chromosomes. MECP2 binding to DNA influencesmRNA splicingevents. MECP2 also appears to function inDNA repairprocesses.MECP2-/+deficient female mice have elevated rates of cell death when exposed to DNA damaging agents and are prone to earlysenescence.[54]
Interactive pathway map
[edit]Aninteractive pathway map of Rett syndromehas been published.[55]
Diagnosis
[edit]
Prior to the discovery of a genetic cause, Rett syndrome had been designated as apervasive developmental disorderby theDiagnostic and Statistical Manual of Mental Disorders(DSM), together with theautism spectrum disorders.Some argued against this conclusive assignment because RTT resembles non-autistic disorders such asfragile X syndrome,tuberous sclerosis,orDown syndromethat also exhibit autistic features.[56] After research proved the molecular mechanism, in 2013 theDSM-5removed the syndrome altogether from classification as a mental disorder.[57]
Rett syndrome diagnosis involves close observation of the child's growth and development to observe any abnormalities in regards to developmental milestones.[58]A diagnosis is considered when decreased head growth is observed. Conditions with similar symptoms must first be ruled out.[58]
There are certain criteria that must be met for the diagnosis. A blood test can rule in or rule out the presence of the MECP2 mutation, however, this mutation is present in other conditions as well.[59]
For a classic diagnosis, all four criteria for ruling in a diagnosis must be met, as well as the two criteria for ruling out a diagnosis. Supportive criteria may also be present, but are not required for diagnosis. For an atypical or variant diagnosis, at least two of the four criteria for ruling in the diagnosis must be met, as well as five of the eleven supportive criteria. A period of symptom regression followed by recovery or symptom stabilization must also occur.[59]Children are often misdiagnosed as having autism, cerebral palsy, or another form of developmental delay. A positive test for the MECP2 mutation is not enough to make a diagnosis.[59]
Ruling in[59]
- Decreased or loss of use of fine motor skills
- Decreased or loss of verbal speech
- Abnormalities during gait
- Repetitive hand movements such as wringing/squeezing or clapping/tapping
Ruling out[59]
- Traumatic or anoxic/hypoxic brain injury, neurometabolic disease, or severe infection that may better explain symptoms
- Abnormal psychomotor development during the first six months of life
Supportive criteria[59]
- Breathing disturbances when awake
- Bruxism while awake
- Impaired sleep pattern
- Abnormal muscle tone
- Peripheral vasomotor disturbances
- Scoliosis/kyphosis
- Growth retardation
- Small cold hands and feet
- Inappropriate laughing/screaming spells
- Diminished response to pain
- Intense eye communication (eye pointing)
Differential diagnosis
[edit]Signs of Rett syndrome that are similar toautism:[60][61]
- screaming fits
- inconsolable crying
- avoidance of eye contact
- lack of social/emotional reciprocity
- markedly impaired use of nonverbal behaviors to regulate social interaction
- loss of speech
- sensory problems
- sleep regression
Signs of Rett syndrome that are also present incerebral palsy:[62][63]
- possible short stature, sometimes with unusual body proportions because of difficulty walking ormalnutritioncaused bydifficulty swallowing
- hypotonia
- delayed or absent ability to walk
- gait/movement difficulties
- ataxia
- microcephalyin some - abnormally small head, poor head growth
- gastrointestinal problems
- some forms ofspasticity
- chorea- spasmodic movements of hand or facial muscles
- dystonia
- bruxism– grinding of teeth
Treatment
[edit]There is no cure for Rett syndrome.[5]Treatment is directed towards improving function and addressing symptoms.[5]A multi-disciplinary team approach is typically used to treat the person throughout life. This team may include aprimary care physician,physical therapist, occupational therapist, speech-language pathologist, nutritionist, and support services in academic and occupational settings. Some children may require special equipment and aids such as braces to arrest scoliosis, splints to modify hand movements, and nutritional programs to help them maintain adequate weight.[5]
Because of the increased risk of sudden cardiac death, whenlong QT syndromeis found on an annual screening EKG it is treated with an anti-arrhythmic such as abeta-blocker.There is some evidence thatphenytoinmay be more effective than a beta-blocker.[64]
While medicinal interventions to mitigate breathing challenges in children with Rett Syndrome (RTT) are still being developed,[65]children with RTT may be prescribed rebreathing techniques (e.g., rebreathing masks), oxygen delivery, or non-invasive ventilation as preventative or rescue breathing treatments.[citation needed]High oxidative stress levels in individuals with RTT have exacerbated effects on their cardiorespiratory health and functionality,[65]dramatically increasing the risk for sudden cardiac death—an anomaly that has an associated 300x increased occurrence risk in children with Rett Syndrome.[66]Due to this, it is vital to closely monitor atypical breathing behaviors in children with RTT, making sure to effectively use lifesaving respiratory improvement devices and strategies as prescribed.[67]
Prescribed treatment methods may vary depending on the breathing characteristic phenotype expressed by the child. Physicians have identified three major RTT breathing phenotypes; forceful breathers, feeble breathers, and apneustic breathers.[68]For forceful breathers, for example, rebreathing masks may be used while the child is awake.[68]
Therapeutic
[edit]Trofinetide
[edit]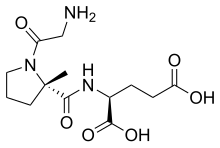
Trofinetide,sold under the brand name Daybue, is amedicationused for the treatment of Rett syndrome.[69]It is takenby mouth.[69]
The most common adverse reactions includediarrheaandvomiting.[70]
Trofinetide was approved for medical use in the United States in March 2023.[69][70][71][72]The USFood and Drug Administration(FDA) considers it to be afirst-in-class medication.[73]Prognosis
[edit]
Male fetuses with the disorder rarely survive to term. Because the disease-causing gene is located on the X chromosome, a female born with an MECP2 mutation on her Xchromosomehas another X chromosome with an ostensibly normal copy of the same gene, while a male with the mutation on his X chromosome has no other X chromosome, only a Y chromosome; thus, he has no normal gene. Without a normal gene to provide normal proteins in addition to the abnormal proteins caused by a MECP2 mutation, the XYkaryotypemale fetus is unable to slow the development of the disease, hence the failure of many male fetuses with a MECP2 mutation to survive to term.[citation needed]Males with pathogenicMECP2mutations usually die within the first 2 years from severeencephalopathy,unless they have one or more extra X chromosomes, or havesomatic mosaicism.
Females with a MECP2 mutation, however, have a non-mutant chromosome that provides them enough normalproteinto survive longer. Research shows that males with Rett syndrome may result fromKlinefelter's syndrome,in which the male has an XXY karyotype.[74]Thus, a non-mutantMECP2gene is necessary for a Rett's-affected embryo to survive in most cases, and the embryo, male or female, must have another X chromosome.
There have, however, been several cases of 46,XY karyotype males with a MECP2 mutation (associated with classical Rett syndrome in females) carried to term, who were affected by neonatal encephalopathy and died before 2 years of age.[75]The incidence of Rett syndrome in males is unknown, partly owing to the low survival of male fetuses with the Rett syndrome-associated MECP2 mutations, and partly to differences between signs caused by MECP2 mutations and those caused by Rett's.[75]
Females can live up to 40 years or more. Laboratory studies on Rett syndrome may show abnormalities such as:
- EEGabnormalities from 2 years of age
- atypical brainglycolipids
- elevated CSF levels ofbeta-endorphinandglutamate
- reduction ofsubstance P
- decreased levels of CSF nerve growth factors
A high proportion of deaths are abrupt, but most have no identifiable cause; in some instances death is the result most likely of:
- spontaneous brainstem dysfunction
- cardiac arrest,likely due tolong QT syndrome,ventricular tachycardiaor other arrhythmias[76]
- seizures
- gastric perforation
History
[edit]Andreas Rett,a pediatrician in Vienna Austria, first described the condition in 1966.[5][77]As his writings were in German, they did not become widely known in the English-speaking world.[7]Bengt Hagberg, a Swedish pediatrician, published an English article in 1983 and named the condition after Rett.[7]In 1999, Lebanese-American physicianHuda Zoghbidiscovered the mutation that causes the condition.[7][8]
Research
[edit]Gene therapyis under study in animal models to achieve regulated expression of a normal MECP2 gene.[5]In March 2022, Taysha Gene Therapies announced that they had received Clinical Trial Application (CTA) approval from Health Canada for a clinical trial of theirinvestigational gene therapyfor adult females with Rett Syndrome.[78]
References
[edit]- ^Davis AS (25 October 2010).Handbook of Pediatric Neuropsychology.Springer Publishing Company.p. 703.ISBN978-0826157362.Archivedfrom the original on 5 November 2017.
Rett initially called this syndrome cerebroaatrophic hyperammonemia, but the elevated ammonia levels in the bloodstream were later found to be only rarely associated with this condition (can Acker, Loncola, & Can Acker, 2005).
- ^Percy A (2014)."Rett Syndrome: Coming to Terms with Treatment".Advances in Neuroscience.2014:1–20.doi:10.1155/2014/345270.
- ^"MeSH Browser".meshb.nlm.nih.gov.Archivedfrom the original on 4 December 2020.Retrieved22 October2019.
- ^abcdefghijklm"Rett syndrome".Genetics Home Reference.December 2013.Archivedfrom the original on 14 October 2017.Retrieved14 October2017.
- ^abcdefghijklmnopqrstuvwxyzaaabacadaeafagahaiajakalamanaoapaq"Rett Syndrome Fact Sheet".National Institute of Neurological Disorders and Stroke.Archivedfrom the original on 14 October 2017.Retrieved14 October2017.
- ^"Rett Syndrome".NORD (National Organization for Rare Disorders).2015.Archivedfrom the original on 19 February 2017.Retrieved14 October2017.
- ^abcdPercy A (January 2014)."The American History of Rett Syndrome".Pediatric Neurology.50(1): 1–3.doi:10.1016/j.pediatrneurol.2013.08.018.PMC3874243.PMID24200039.
- ^abAmir R, Van den Veyver I, Wan M, Tran C, Francke U, Zoghbi H (1999). "Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2".Nature Genetics.23(2): 185–8.doi:10.1038/13810.PMID10508514.S2CID3350350.
- ^Neul Jl, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. (Rettsearch Consortium) (2010)."Rett syndrome: Revised diagnostic criteria and nomenclature".Annals of Neurology.68(6): 944–50.doi:10.1002/ana.22124.PMC3058521.PMID21154482.
- ^Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, et al. (11 July 2008)."FOXG1 is Responsible for the Congenital Variant of Rett Syndrome".The American Journal of Human Genetics.83(1): 89–93.doi:10.1016/j.ajhg.2008.05.015.PMC2443837.PMID18571142.
- ^Zappella M (1992). "The rett girls with preserved speech".Brain and Development.14(2): 98–101.doi:10.1016/S0387-7604(12)80094-5.PMID1621933.S2CID4782923.
- ^Skjeldal OH, Von Tetzchner S, Jacobsen K, Smith L, Heiberg A (2007). "Rett Syndrome - Distribution of Phenotypes with Special Attention to the Preserved Speech Variant".Neuropediatrics.26(2): 87.doi:10.1055/s-2007-979732.PMID7566462.S2CID260243402.
- ^Sørensen E, Viken B (20 February 1995). "[Rett syndrome a developmental disorder. Presentation of a variant with preserved speech]".Tidsskrift for den Norske Laegeforening(in Norwegian).115(5): 588–590.ISSN0029-2001.PMID7900110.
- ^Zappella M (1997). "The preserved speech variant of the Rett complex: A report of 8 cases".European Child & Adolescent Psychiatry.6(Suppl 1): 23–5.PMID9452915.
- ^Renieri A, Mari F, Mencarelli M, Scala E, Ariani F, Longo I, et al. (March 2009). "Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant)".Brain and Development.31(3): 208–16.doi:10.1016/j.braindev.2008.04.007.PMID18562141.S2CID6223422.
- ^Buoni S, Zannolli R, De Felice C, De Nicola A, Guerri V, Guerra B, et al. (May 2010). "EEG features and epilepsy in MECP2-mutated patients with the Zappella variant of Rett syndrome".Clinical Neurophysiology.121(5): 652–7.doi:10.1016/j.clinph.2010.01.003.PMID20153689.S2CID12976926.
- ^Huppke P, Held M, Laccone F, Hanefeld F (2003). "The spectrum of phenotypes in females with Rett Syndrome".Brain and Development.25(5): 346–51.doi:10.1016/S0387-7604(03)00018-4.PMID12850514.S2CID9566219.
- ^Gillberg d (1997). "Communication in Rett syndrome complex".European Child & Adolescent Psychiatry.6(Suppl 1): 21–2.PMID9452914.
- ^Zappella M, Gillberg C, Ehlers S (1998). "The preserved speech variant: A subgroup of the Rett complex: A clinical report of 30 cases".Journal of Autism and Developmental Disorders.28(6): 519–26.doi:10.1023/A:1026052128305.PMID9932238.S2CID22152062.
- ^abNeul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. (2010)."Rett Syndrome: Revised Diagnostic Criteria and Nomenclature".Annals of Neurology.68(6): 944–950.doi:10.1002/ana.22124.ISSN0364-5134.PMC3058521.PMID21154482.
- ^Fahmi M, Yasui G, Seki K, Katayama S, Kaneko-Kawano T, Inazu T, et al. (2019)."In Silico Study of Rett Syndrome Treatment-Related Genes, MECP2, CDKL5, and FOXG1, by Evolutionary Classification and Disordered Region Assessment".International Journal of Molecular Sciences.20(22): 5593.doi:10.3390/ijms20225593.ISSN1422-0067.PMC6888432.PMID31717404.
- ^Cutri-French C, Armstrong D, Saby J, Gorman C, Lane J, Fu C, et al. (2020)."Comparison of core features in four Developmental Encephalopathies in the Rett Natural History Study".Annals of Neurology.88(2): 396–406.doi:10.1002/ana.25797.ISSN0364-5134.PMC8882337.PMID32472944.
- ^Guy J, Gan J, Selfridge J, Cobb S, Bird A (2007)."Reversal of Neurological Defects in a Mouse Model of Rett Syndrome".Science.315(5815): 1143–7.Bibcode:2007Sci...315.1143G.doi:10.1126/science.1138389.PMC7610836.PMID17289941.S2CID25172134.
- ^Trappe R, Laccone F, Cobilanschi J, Meins M, Huppke P, Hanefeld F, et al. (2001)."MECP2 Mutations in Sporadic Cases of Rett Syndrome Are Almost Exclusively of Paternal Origin".The American Journal of Human Genetics.68(5): 1093–101.doi:10.1086/320109.PMC1226090.PMID11309679.
- ^"Rett Syndrome - Symptoms, Causes, Treatment | NORD".rarediseases.org.Retrieved5 February2024.
- ^Zoghbi HY, Van Den Veyver RE, Wan IB, Tran M, Francke CQ, Zoghbi U (1999). "Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2".Nature Genetics.23(2): 185–8.doi:10.1038/13810.PMID10508514.S2CID3350350.
- ^"Rett syndrome".Genetics Home Reference.Archivedfrom the original on 27 July 2016.Retrieved29 May2016.
- ^Merwick A, O'Brien M, Delanty N (2012)."Complex single gene disorders and epilepsy".Epilepsia.53(s4): 81–91.doi:10.1111/j.1528-1167.2012.03617.x.ISSN1528-1167.PMID22946725.S2CID37226510.
- ^Zoghbi HY, Milstien S, Butler IJ, Smith EO, Kaufman S, Glaze DG, et al. (1989). "Cerebrospinal fluid biogenic amines and biopterin in Rett syndrome".Annals of Neurology.25(1): 56–60.doi:10.1002/ana.410250109.PMID2913929.S2CID351243.
- ^abRoux JC, Villard L (2009). "Biogenic Amines in Rett Syndrome: The Usual Suspects".Behavior Genetics.40(1): 59–75.doi:10.1007/s10519-009-9303-y.PMID19851857.S2CID20352177.
- ^Hokfelt T, Martensson R, Bjorklund A, Kleinau S, Goldstein M (1984). "Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain". In Bjorklund A, Hokfelt T (eds.).Handbook of Chemical Neuroanatomy.Classical Transmitters in the CNS, Part I. Vol. 2. New York: Elsevier. pp. 277–379.
- ^Berridge CW, Waterhouse BD (2003). "The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes".Brain Research Reviews.42(1): 33–84.doi:10.1016/S0165-0173(03)00143-7.PMID12668290.S2CID477754.
- ^abTaneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB (2009)."Pathophysiology of Locus Ceruleus Neurons in a Mouse Model of Rett Syndrome".Journal of Neuroscience.29(39): 12187–95.doi:10.1523/JNEUROSCI.3156-09.2009.PMC2846656.PMID19793977.
- ^abcdRoux JC, Panayotis N, Dura E, Villard L (2009). "Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice".Journal of Neuroscience Research.88(7): 1500–9.doi:10.1002/jnr.22312.PMID19998492.S2CID3404695.
- ^Björklund A, Lindvall O (1984). "Dopamine-containing systems in the CNS". In Björklund A, Hökfelt T (eds.).Handbook of Chemical Neuroanatomy.Classical Transmitters in the CNS, Part l. Vol. 2. New York: Elsevier. pp. 55–122.
- ^Hokfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M (1984). "Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain". In Björklund A, Hökfelt T (eds.).Handbook of Chemical Neuroanatomy.Classical Transmitters in the CNS, Part I. Vol. 2. New York: Elsevier. pp. 277–379.
- ^Björklund A, Dunnett SB (2007). "Dopamine neuron systems in the brain: An update".Trends in Neurosciences.30(5): 194–202.doi:10.1016/j.tins.2007.03.006.PMID17408759.S2CID14239716.
- ^Parent A, Hazrati LN (1995). "Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop".Brain Research Reviews.20(1): 91–127.doi:10.1016/0165-0173(94)00007-C.PMID7711769.S2CID28252990.
- ^Gerfen CR (2000). "Molecular effects of dopamine on striatal-projection pathways".Trends in Neurosciences.23(10 Suppl): S64–70.doi:10.1016/S1471-1931(00)00019-7.PMID11052222.S2CID3965480.
- ^Lees AJ, Hardy J, Revesz T (2009). "Parkinson's disease".The Lancet.373(9680): 2055–66.doi:10.1016/S0140-6736(09)60492-X.PMID19524782.S2CID42608600.
- ^Dauer W, Przedborski S (2003)."Parkinson's Disease".Neuron.39(6): 889–909.doi:10.1016/S0896-6273(03)00568-3.PMID12971891.S2CID10400095.
- ^Jenner P (2009). "Functional models of Parkinson's disease: A valuable tool in the development of novel therapies".Annals of Neurology.64:S16–29.doi:10.1002/ana.21489.PMID19127585.S2CID26065287.
- ^Fitzgerald PM, Jankovic J, Percy AK (1990). "Rett syndrome and associated movement disorders".Movement Disorders.5(3): 195–202.doi:10.1002/mds.870050303.PMID2388636.S2CID43376602.
- ^Neul JL, Zoghbi HY (2004). "Rett Syndrome: A Prototypical Neurodevelopmental Disorder".The Neuroscientist.10(2): 118–28.doi:10.1177/1073858403260995.PMID15070486.S2CID9617631.
- ^Segawa M (2005). "Early motor disturbances in Rett syndrome and its pathophysiological importance".Brain and Development.27:S54–S58.doi:10.1016/j.braindev.2004.11.010.PMID16182486.S2CID30218744.
- ^Guy J, Hendrich B, Holmes M, Martin JE, Bird A (2001). "A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome".Nature Genetics.27(3): 322–6.doi:10.1038/85899.hdl:1842/727.PMID11242117.S2CID8698208.
- ^Chen RZ, Akbarian S, Tudor M, Jaenisch R (2001). "Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice".Nature Genetics.27(3): 327–31.doi:10.1038/85906.PMID11242118.S2CID24979562.
- ^Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. (1998). "Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex".Nature.393(6683): 386–9.Bibcode:1998Natur.393..386N.doi:10.1038/30764.PMID9620804.S2CID4427745.
- ^Cheval H, Guy J, Merusi C, De Sousa D, Selfridge J,Bird A(2012)."Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows".Human Molecular Genetics.21(17): 3806–14.doi:10.1093/hmg/dds208.PMC3412380.PMID22653753.

- ^Ricceri L, De Filippis B, Laviola G (2008). "Mouse models of Rett syndrome: From behavioural phenotyping to preclinical evaluation of new therapeutic approaches".Behavioural Pharmacology.19(5–6): 501–17.doi:10.1097/FBP.0b013e32830c3645.PMID18690105.S2CID33364486.
- ^Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, et al. (2009)."Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities".Proceedings of the National Academy of Sciences.106(51): 21966–71.Bibcode:2009PNAS..10621966S.doi:10.1073/pnas.0912257106.JSTOR40536204.PMC2799790.PMID20007372.
- ^abcdPanayotis N, Pratte M, Borges-Correia A, Ghata A, Villard L, Roux JC (2011). "Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse".Neurobiology of Disease.41(2): 385–97.doi:10.1016/j.nbd.2010.10.006.PMID20951208.S2CID25414717.
- ^Sun YE, Wu H (2006)."The Ups and Downs of BDNF in Rett Syndrome".Neuron.49(3): 321–3.doi:10.1016/j.neuron.2006.01.014.PMID16446133.
- ^abSharifi O, Yasui DH (2021)."The Molecular Functions of MeCP2 in Rett Syndrome Pathology".Frontiers in Genetics.12:624290.doi:10.3389/fgene.2021.624290.PMC8102816.PMID33968128.
- ^Ehrhart F, Coort SL, Cirillo E, Smeets E, Evelo CT, Curfs LM (25 November 2016)."Rett syndrome – biological pathways leading from MECP2 to disorder phenotypes".Orphanet Journal of Rare Diseases.11(1): 158.doi:10.1186/s13023-016-0545-5.PMC5123333.PMID27884167.
- ^Tsai LY (1992)."Is Rett syndrome a subtype of pervasive developmental disorders?"(PDF).Journal of Autism and Developmental Disorders.22(4): 551–61.doi:10.1007/BF01046327.hdl:2027.42/44607.PMID1483976.S2CID17817425.Archivedfrom the original on 29 August 2021.Retrieved20 April2018.
- ^Abbeduto L, Ozonoff S, Thurman AJ, McDuffie A, Schweitzer J (18 March 2014). Hales R, Yudofsky S, Robert LW (eds.).Chapter 8. Neurodevelopmental Disorders, The American Psychiatric Publishing Textbook of Psychiatry(6 ed.). Arlington, VA: American Psychiatric Publishing.doi:10.1176/appi.books.9781585625031.rh08.ISBN978-1-58562-444-7.S2CID241966275.
- ^ab"Rett syndrome Tests and diagnosis".Mayo Clinic.Archivedfrom the original on 30 October 2017.
- ^abcdef"About Rett syndrome - Rett Syndrome Diagnosis".rettsyndrome.org.International Rett Syndrome Foundation.Archivedfrom the original on 29 October 2017.Retrieved10 May2020.
- ^"Seven Disorders Closely Related to Autism".Autism Research Institute.Retrieved5 February2024.
- ^Neul JL (2012)."The relationship of Rett syndrome and MECP2 disorders to autism".Dialogues in Clinical Neuroscience.14(3): 253–262.doi:10.31887/DCNS.2012.14.3/jneul.ISSN1294-8322.PMC3513680.PMID23226951.
- ^"Rett Syndrome | Rady Children's Hospital".rchsd.org.Retrieved5 February2024.
- ^"Cerebral Palsy Misdiagnosis".Cerebral Palsy Guidance.Retrieved5 February2024.
- ^McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, et al. (14 December 2011)."Pathogenesis of Lethal Cardiac Arrhythmias in Mecp2 Mutant Mice: Implication for Therapy in Rett Syndrome".Science Translational Medicine.3(113): 113ra125.doi:10.1126/scitranslmed.3002982.ISSN1946-6234.PMC3633081.PMID22174313.
- ^abMackay J, Downs J, Wong K, Heyworth J, Epstein A, Leonard H (2017)."Autonomic breathing abnormalities in Rett syndrome: caregiver perspectives in an international database study".Journal of Neurodevelopmental Disorders.9:15.doi:10.1186/s11689-017-9196-7.ISSN1866-1947.PMC5410057.PMID28465761.
- ^Kyle SM, Vashi N,Justice MJ(February 2018)."Rett syndrome: a neurological disorder with metabolic components".Open Biology.8(2): 170216.doi:10.1098/rsob.170216.ISSN2046-2441.PMC5830535.PMID29445033.
- ^De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G, et al. (April 2012)."Subclinical myocardial dysfunction in Rett syndrome".European Heart Journal: Cardiovascular Imaging.13(4): 339–345.doi:10.1093/ejechocard/jer256.ISSN2047-2412.PMID22113206.Archivedfrom the original on 29 November 2021.Retrieved29 November2021.
- ^abSmeets EE, Julu PO, Waardenburg Dv, Engerström IW, Hansen S, Apartopoulos F, et al. (1 November 2006)."Management of a severe forceful breather with Rett Syndrome using carbogen".Brain and Development.28(10): 625–632.doi:10.1016/j.braindev.2006.04.010.ISSN0387-7604.PMID16765005.S2CID15545729.Archivedfrom the original on 1 October 2022.Retrieved29 November2021.
- ^abc"Daybue- trofinetide solution".DailyMed.29 March 2023.Archivedfrom the original on 2 July 2023.Retrieved20 November2023.
- ^ab"FDA approves first treatment for Rett Syndrome".U.S.Food and Drug Administration(FDA).13 March 2023.Archivedfrom the original on 13 March 2023.Retrieved13 March2023.
 This article incorporates text from this source, which is in thepublic domain.
This article incorporates text from this source, which is in thepublic domain.
- ^Keam SJ (June 2023)."Trofinetide: First Approval".Drugs.83(9): 819–824.doi:10.1007/s40265-023-01883-8.PMID37191913.S2CID258715933.
- ^"Drug Trials Snapshots: Daybue".U.S.Food and Drug Administration(FDA).12 March 2023.Retrieved19 July2024.
 This article incorporates text from this source, which is in thepublic domain.
This article incorporates text from this source, which is in thepublic domain.
- ^New Drug Therapy Approvals 2023(PDF).U.S.Food and Drug Administration(FDA)(Report). January 2024.Archivedfrom the original on 10 January 2024.Retrieved9 January2024.
- ^Schwartzman JS, Bernardino A, Nishimura A, Gomes RR, Zatz M (2001). "Rett Syndrome in a Boy with a 47,XXY Karyotype Confirmed by a Rare Mutation in the MECP2 Gene".Neuropediatrics.32(3): 162–4.doi:10.1055/s-2001-16620.PMID11521215.S2CID260240039.
- ^abHardwick SA, Reuter K, Williamson SL, Vasudevan V, Donald J, Slater K, et al. (2007)."Delineation of large deletions of the MECP2 gene in Rett syndrome patients, including a familial case with a male proband".European Journal of Human Genetics.15(12): 1218–29.doi:10.1038/sj.ejhg.5201911.PMID17712354.
- ^Acampa M, Guideri F (May 2006)."Cardiac disease and Rett syndrome".Archives of Disease in Childhood.91(5): 440–443.doi:10.1136/adc.2005.090290.ISSN1468-2044.PMC2082747.PMID16632674.
- ^Rett A(10 September 1966). "[On an unusual brain atrophy syndrome in hyperammonemia in childhood]".Wiener Medizinische Wochenschrift(in German).116(37): 723–726.ISSN0043-5341.PMID5300597.
- ^"Taysha Gene Therapies Announces Initiation of Clinical Development of TSHA-102 in Rett Syndrome".Taysha Gene Therapies.March 2022.Archivedfrom the original on 30 March 2022.Retrieved6 May2022.
