Acid strength
Acid strengthis the tendency of anacid,symbolised by thechemical formula,to dissociate into aproton,,and ananion,.Thedissociationorionizationof a strong acid in solution is effectively complete, except in its most concentrated solutions.
Examples ofstrong acidsarehydrochloric acid,perchloric acid,nitric acidandsulfuric acid.
A weak acid is only partially dissociated, or is partly ionized in water with both the undissociated acid and its dissociation products being present, in solution, inequilibriumwith each other.
Acetic acid() is an example of a weak acid. The strength of a weak acid is quantified by itsacid dissociation constant,value.
The strength of a weakorganicacid may depend on substituent effects. The strength of aninorganicacid is dependent on theoxidation statefor the atom to which the proton may be attached. Acid strength is solvent-dependent. For example,hydrogen chlorideis a strong acid in aqueous solution, but is a weak acid when dissolved inglacial acetic acid.
Measures of acid strength
The usual measure of the strength of an acid is itsacid dissociation constant(), which can bedetermined experimentallybytitrationmethods. Stronger acids have a largerand a smaller logarithmic constant () than weaker acids. The stronger an acid is, the more easily it loses a proton,.Two key factors that contribute to the ease ofdeprotonationare thepolarityof thebond and the size of atom A, which determine the strength of thebond. Acid strengths also depend on the stability of the conjugate base.
While thevalue measures the tendency of an acidic solute to transfer a proton to a standard solvent (most commonly water orDMSO), the tendency of an acidic solvent to transfer a proton to a reference solute (most commonly a weakanilinebase) is measured by itsHammett acidity function,thevalue. Although these two concepts of acid strength often amount to the same general tendency of a substance to donate a proton, theandvalues are measures of distinct properties and may occasionally diverge. For instance, hydrogen fluoride, whether dissolved in water (= 3.2) or DMSO (= 15), hasvalues indicating that it undergoes incomplete dissociation in these solvents, making it a weak acid. However, as the rigorously dried, neat acidic medium, hydrogen fluoride has anvalue of –15,[1]making it a more strongly protonating medium than 100% sulfuric acid and thus, by definition, asuperacid.[2](To prevent ambiguity, in the rest of this article, "strong acid" will, unless otherwise stated, refer to an acid that is strong as measured by itsvalue (< –1.74). This usage is consistent with the common parlance of most practicingchemists.)
When the acidic medium in question is a dilute aqueous solution, theis approximately equal to thepHvalue, which is a negative logarithm of the concentration of aqueousin solution. The pH of a simple solution of an acid in water is determined by bothand the acid concentration. For weak acid solutions, it depends on thedegree of dissociation,which may be determined by an equilibrium calculation. For concentrated solutions of acids, especially strong acids for which pH < 0, thevalue is a better measure of acidity than the pH.
Strong acids

Astrong acidis an acid that dissociates according to the reaction
where S represents a solvent molecule, such as a molecule of water ordimethyl sulfoxide(DMSO), to such an extent that the concentration of the undissociated speciesis too low to be measured. For practical purposes a strong acid can be said to be completely dissociated. An example of a strong acid isperchloric acid.
- (in aqueous solution)
Any acid with avalue which is less than about -2 is classed as a strong acid. This results from the very highbuffer capacityof solutions with apH valueof 1 or less and is known as theleveling effect.[3]
The following are strong acids in aqueous and dimethyl sulfoxide solution. The values of,cannot be measured experimentally. The values in the following table are average values from as many as 8 different theoretical calculations.
EstimatedpKavalues[4] Acid Formula in water in DMSO Hydrochloric acid HCl −5.9 ± 0.4 −2.0 ± 0.6 Hydrobromic acid HBr −8.8 ± 0.8 −6.8 ± 0.8 Hydroiodic acid HI −9.5 ± 1 −10.9 ± 1 Triflic acid H[CF3SO3] −14 ± 2 −14 ± 2 Perchloric acid H[ClO4] −15 ± 2 −15 ± 2
Also, in water
- Nitric acid= −1.6[5]
- Sulfuric acid(first dissociation only,≈ −3)[6]: (p. 171)
The following can be used as protonators inorganic chemistry
Sulfonic acids,such asp-toluenesulfonic acid(tosylic acid) are a class of strong organicoxyacids.[7]Some sulfonic acids can be isolated as solids.Polystyrenefunctionalized intopolystyrene sulfonateis an example of a substance that is a solid strong acid.
Weak acids

A weak acid is a substance that partially dissociates, or partly ionizes when it is dissolved in a solvent. In solution there is an equilibrium between the acid,,and the products of dissociation.
The solvent (e.g. water) is omitted from this expression when its concentration is effectively unchanged by the process of acid dissociation. The strength of a weak acid can be quantified in terms of adissociation constant,,defined as follows, wheresignifies the concentration of a chemical moiety, X.
When a numerical value ofis known it can be used to determine the extent of dissociation in a solution with a given concentration of the acid,,by applying the law ofconservation of mass.
whereis the value of theanalytical concentrationof the acid. When all the quantities in this equation are treated as numbers, ionic charges are not shown and this becomes aquadratic equationin the value of the hydrogen ion concentration value,.
This equation shows that the pH of a solution of a weak acid depends on both itsvalue and its concentration. Typical examples of weak acids includeacetic acidandphosphorous acid.An acid such asoxalic acid() is said to bedibasicbecause it can lose two protons and react with two molecules of a simple base.Phosphoric acid() is tribasic.
For a more rigorous treatment of acid strength seeacid dissociation constant.This includes acids such as the dibasic acidsuccinic acid,for which the simple method of calculating the pH of a solution, shown above, cannot be used.
Experimental determination
The experimental determination of avalue is commonly performed by means of atitration.[8]A typical procedure would be as follows. A quantity of strong acid is added to a solution containing the acid or a salt of the acid, to the point where the compound is fully protonated. The solution is then titrated with a strong base
until only the deprotonated species,,remains in solution. At each point in the titration pH is measured using aglass electrodeand apH meter.The equilibrium constant is found by fitting calculated pH values to the observed values, using the method ofleast squares.
Conjugate acid/base pair
It is sometimes stated that "the conjugate of a weak acid is a strong base". Such a statement is incorrect. For example, acetic acid is a weak acid which has a= 1.75 x 10−5.Its conjugate base is theacetateion withKb= 10−14/Ka= 5.7 x 10−10(from the relationshipKa×Kb= 10−14), which certainly does not correspond to a strong base. The conjugate of a weak acid is often a weak base andvice versa.
Acids in non-aqueous solvents
The strength of an acid varies from solvent to solvent. An acid which is strong in water may be weak in a less basic solvent, and an acid which is weak in water may be strong in a more basic solvent. According toBrønsted–Lowry acid–base theory,the solvent S can accept a proton.
For example, hydrochloric acid is a weak acid in solution in pureacetic acid,,which is more acidic than water.
The extent of ionization of thehydrohalic acidsdecreases in the order.Acetic acid is said to be adifferentiating solventfor the three acids, while water is not.[6]: (p. 217)
An important example of a solvent which is more basic than water isdimethyl sulfoxide,DMSO,.A compound which is a weak acid in water may become a strong acid in DMSO.Acetic acidis an example of such a substance. An extensive bibliography ofvalues in solution in DMSO and other solvents can be found atAcidity–Basicity Data in Nonaqueous Solvents.
Superacidsare strong acids even in solvents of low dielectric constant. Examples of superacids arefluoroantimonic acidandmagic acid.Some superacids can be crystallised.[9]They can also quantitatively stabilizecarbocations.[10]
Lewis acidsreacting with Lewis bases in gas phase and non-aqueous solvents have been classified in theECW model,and it has been shown that there is no one order of acid strengths.[11]The relative acceptor strength of Lewis acids toward a series of bases, versus other Lewis acids, can be illustrated byC-B plots.[12][13]It has been shown that to define the order of Lewis acid strength at least two properties must be considered. For the qualitativeHSAB theorythe two properties are hardness and strength while for the quantitativeECW modelthe two properties are electrostatic and covalent.
Factors determining acid strength
The inductive effect
In organic carboxylic acids, an electronegative substituent can pull electron density out of an acidic bond through theinductive effect,resulting in a smallervalue. The effect decreases, the further the electronegative element is from the carboxylate group, as illustrated by the following series ofhalogenatedbutanoic acids.
| Structure | Name | pKa |
|---|---|---|
 |
2-chlorobutanoic acid | 2.86 |
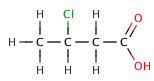 |
3-chlorobutanoic acid | 4.0 |
 |
4-chlorobutanoic acid | 4.5 |
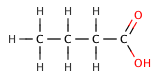 |
butanoic acid | 4.5 |
Effect of oxidation state
In a set ofoxoacidsof an element,values decrease with the oxidation state of the element. The oxoacids of chlorine illustrate this trend.[6]: (p. 171)
| Structure | Name | Oxidation state |
pKa |
|---|---|---|---|
 |
perchloric acid | 7 | -8† |
 |
chloric acid | 5 | -1 |
| chlorous acid | 3 | 2.0 | |
 |
hypochlorous acid | 1 | 7.53 |
† theoretical
References
- ^Liang, Joan-Nan Jack (1976).The Hammett Acidity Function for Hydrofluoric Acid and some related Superacid Systems (Ph.D. Thesis)(PDF).Hamilton, Ontario: McMaster University. p. 94.
- ^Miessler G.L. and Tarr D.A.Inorganic Chemistry(2nd ed., Prentice-Hall 1998, p.170)ISBN0-13-841891-8
- ^Porterfield, William W.Inorganic Chemistry(Addison-Wesley 1984) p.260ISBN0-201-05660-7
- ^Trummal, Aleksander; Lipping, Lauri; Kaljurand, Ivari; Koppel, Ilmar A.; Leito, Ivo (2016). "Acidity of strong acids in water and dimethyl sulfoxide".J. Phys. Chem. A.120(20): 3663–3669.Bibcode:2016JPCA..120.3663T.doi:10.1021/acs.jpca.6b02253.PMID27115918.S2CID29697201.
- ^Bell, R. P. (1973),The Proton in Chemistry(2nd ed.), Ithaca, NY: Cornell University Press
- ^abcHousecroft, C. E.; Sharpe, A. G. (2004).Inorganic Chemistry(2nd ed.). Prentice Hall.ISBN978-0-13-039913-7.
- ^abGuthrie, J.P. (1978)."Hydrolysis of esters of oxy acids: pKavalues for strong acids ".Can. J. Chem.56(17): 2342–2354.doi:10.1139/v78-385.
- ^Martell, A.E.; Motekaitis, R.J. (1992).Determination and Use of Stability Constants.Wiley.ISBN0-471-18817-4.Chapter 4: Experimental Procedure for PotentiometricpHMeasurement of Metal Complex Equilibria
- ^Zhang, Dingliang; Rettig, Stephen J.; Trotter, James; Aubke, Friedhelm (1996). "Superacid Anions: Crystal and Molecular Structures of Oxonium Undecafluorodiantimonate(V), [H3O][Sb2F11], Cesium Fluorosulfate, CsSO3F, Cesium Hydrogen Bis(fluorosulfate), Cs[H(SO3F)2], Cesium Tetrakis(fluorosulfato)aurate(III), Cs[Au(SO3F)4], Cesium Hexakis(fluorosulfato)platinate(IV), Cs2[Pt(SO3F)6], and Cesium Hexakis(fluorosulfato)antimonate(V), Cs[Sb(SO3F)6] ".Inorg. Chem.35(21): 6113–6130.doi:10.1021/ic960525l.
- ^George A. Olah,Schlosberg RH (1968). "Chemistry in Super Acids. I. Hydrogen Exchange and Polycondensation of Methane and Alkanes in FSO3H–SbF5( "Magic Acid" ) Solution. Protonation of Alkanes and the Intermediacy of CH5+and Related Hydrocarbon Ions. The High Chemical Reactivity of "Paraffins" in Ionic Solution Reactions ".Journal of the American Chemical Society.90(10): 2726–7.doi:10.1021/ja01012a066.
- ^Vogel G. C.; Drago, R. S. (1996). "The ECW Model".Journal of Chemical Education.73(8): 701–707.Bibcode:1996JChEd..73..701V.doi:10.1021/ed073p701.
- ^Laurence, C. and Gal, J-F. Lewis Basicity and Affinity Scales, Data and Measurement, (Wiley 2010) pp 50-51 ISBN 978-0-470-74957-9
- ^Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases".Journal of Chemical Education.54:612–613.doi:10.1021/ed054p612.The plots shown in this paper used older parameters. Improved E&C parameters are listed inECW model.
External links
- Titration of acids- freeware for data analysisand simulation of potentiometric titration curves






















![{\displaystyle {\ce {H[SbF6]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/81fc97f79d4c3d395f535e093347d7e531a3abdf)
![{\displaystyle {\ce {H[FSO3SbF5]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bc571089e72c64e3866cc0dac118eeabe50708a3)
![{\displaystyle {\ce {H[CHB11Cl11]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/90c05caa0a8732d637a5f63affde47aecf5a8030)
![{\displaystyle {\ce {H[FSO3]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bccda1d1c4b551781d4652eb6014aa363ea80da7)


![{\displaystyle {\ce {[H]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/570fdda794ab5f5775dc52489586e624583bbaa7)
![{\displaystyle K_{a}={\frac {[H^{+}][A^{-}]}{[HA]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/83ee86c6746a584bd7b209324db405b0563af917)

![{\displaystyle {\begin{aligned}T_{H}&=[H]+[HA]\\&=[H]+[A][H]/K_{a}\\&=[H]+[H]^{2}/K_{a}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2d1d8921a76226cabb40caf4153fb742226ac0b6)
![{\displaystyle {\frac {[H]^{2}}{K_{a}}}+[H]-T_{H}=0}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3bcbd6b27c9a030ff81364ef6128a3d3af18d630)







