Acetal

Inorganic chemistry,anacetalis afunctional groupwith the connectivityR2C(OR')2.Here, the R groups can be organic fragments (acarbonatom, with arbitrary other atoms attached to that) orhydrogen,while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal" ) or not (a "mixed acetal" ). Acetals are formed from and convertible toaldehydesorketonesand have the sameoxidation stateat the central carbon, but have substantially differentchemical stabilityandreactivityas compared to the analogouscarbonylcompounds. The central carbon atom has four bonds to it, and is thereforesaturatedand hastetrahedral geometry.
The termketalis sometimes used to identify structures associated withketones(both R groups organic fragments rather than hydrogen) rather thanaldehydesand, historically, the termacetalwas used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on the central carbon).[1]The IUPAC originally deprecated the usage of the word ketal altogether, but has since reversed its decision. However, in contrast to historical usage, ketals are now a subset of acetals, a term that now encompasses both aldehyde- and ketone-derived structures.
If one of the R groups has an oxygen as the first atom (that is, there are more than two oxygens single-bonded to the central carbon), the functional group is instead anorthoester.In contrast to variations of R, both R' groups are organic fragments. If one R' is a hydrogen, the functional group is instead ahemiacetal,while if both are H, the functional group is a ketonehydrateor aldehyde hydrate.
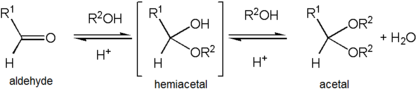
Formation of an acetal occurs when thehydroxylgroup of ahemiacetalbecomesprotonatedand is lost as water. Thecarbocationthat is produced is then rapidly attacked by a molecule ofalcohol.Loss of the proton from the attached alcohol gives the acetal.
Acetals are stable compared to hemiacetals but their formation is a reversibleequilibriumas withesters.As a reaction to create an acetal proceeds, water must be removed from the reaction mixture, for example, with aDean–Stark apparatus,lest ithydrolysethe product back to the hemiacetal. The formation of acetals reduces the total number of molecules present (carbonyl + 2 alcohol → acetal + water) and therefore is generally not favourable with regards toentropy.One situation where it is not entropically unfavourable is when a singlediolmolecule is used rather than two separate alcohol molecules (carbonyl + diol → acetal + water).
Acetalisation and ketalization
[edit]Acetalisation and ketalization are theorganic reactionsthat involve the formation of an acetal (or ketals) from aldehydes and ketones, respectively. These conversions areacidcatalysed.They eliminate water. Since each step is often a rapid equilibrium, the reaction must be driven by removal of water. Methods for removing water includeazeotropic distillationand trapping water with desiccants likealuminium oxideandmolecular sieves.Steps assumed to be involved: protonation of the carbonyl oxygen, addition of the alcohol to the protonated carbonyl, protonolysis of the resultinghemiacetalor hemiketal, and addition of the second alcohol. These steps are illustrated with an aldehyde RCH=O and the alcohol R'OH:
- RCH=O + H+⇌ RCH=OH+
- RCH=OH++ R'OH ⇌ RCH(OH)(OR') + H+
- RCH(OH)(OR') + H+⇌ RC+H(OR') + H2O
- RC+H(OR') + R'OH ⇌ RCH(OR')2+ H+
Another way to avoid the entropic cost is to perform the synthesis by acetal exchange, using a pre-existing acetal-type reagent as the OR'-group donor rather than simple addition of alcohols themselves. One type of reagent used for this method is an orthoester. In this case, water produced along with the acetal product is destroyed when it hydrolyses residual orthoester molecules, and thisside reactionalso produces more alcohol to be used in the main reaction.
Examples
[edit]Sugars
[edit]Since many sugars are polyhydroxy aldehydes and ketones, sugars are a rich source of acetals and ketals. Mostglycosidic bondsincarbohydratesand otherpolysaccharidesare acetal linkages.[2]Celluloseis a ubiquitous example of a polyacetal.
Benzylidene acetalandacetonideas protecting groups used in research of modified sugars.
Chiral derivatives
[edit]Acetals also find application aschiralauxiliaries. Indeed acetals of chiral glycols like, e.g. derivatives of tartaric acid can be asymmetrically opened with high selectivity. This enables the construction of new chiral centers.[3]

Formaldehyde and acetaldehyde
[edit]Formaldehyde forms a rich collection of acetals. This tendency reflects the fact that low molecular weight aldehydes are prone to self-condensation such that the C=O bond is replaced by an acetal. The acetal formed fromformaldehyde(two hydrogens attached to the central carbon) is sometimes called aformal[4]or themethylenedioxygroup. The acetal formed fromacetoneis sometimes called anacetonide.Formaldehyde formsParaldehydeand1,3,5-Trioxane.Polyoxymethylene(POM) plastic, also known as "acetal" or "polyacetal", is a polyacetal (and a polyether), and a polymer offormaldehyde.Acetaldehydeconverts toMetaldehyde.
Unusual acetals
[edit]Phenylsulfonylethylidene(PSE) acetal is an example of arylsulfonyl acetal possessing atypical properties, like resistance to acid hydrolysis which leads to selective introduction and removal of the protective group.[5]
Flavors and fragrances
[edit]1,1-Diethoxyethane(acetaldehyde diethyl acetal), sometimes called simply "acetal", is an important flavouring compound indistilled beverages.[6]Two ketals of ethyl acetoacetate are used in commercial fragrances.[7]Fructone(CH3C(O2C2H4)CH2CO2C2H5), an ethylene glycol ketal, and fraistone (CH3C(O2C2H3CH3)CH2CO2C2H5), an propylene glycol ketal, a commercial fragrances.
Related compounds
[edit]Used in a more general sense, the termX,Y-acetalalso refers to any functional group that consists of a carbon bearing two heteroatomsXandY.For example,N,O-acetal refers to compounds of type R1R2C(OR)(NR'2) (R,R' ≠ H) also known as ahemiaminal etherorAminal,a.k.a. aminoacetal.
S,S-acetal refers to compounds of type R1R2C(SR)(SR') (R,R' ≠ H, also known asthioacetalandthioketals.
See also
[edit]References
[edit]- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "ketals".doi:10.1351/goldbook.K03376
- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "glycosides".doi:10.1351/goldbook.G02661
- ^P.J. Kocieński:Protecting Groups,S. 164–167.
- ^Morrison, Robert T. and Boyd, Robert N., "Organic Chemistry (6th ed)". p683. Prentice-Hall Inc (1992).
- ^Chéry, Florence; Rollin, Patrick; De Lucchi, Ottorino; Cossu, Sergio (2000). "Phenylsulfonylethylidene (PSE) acetals as atypical carbohydrate-protective groups".Tetrahedron Letters.41(14): 2357–2360.doi:10.1016/s0040-4039(00)00199-4.ISSN0040-4039.
- ^Maarse, Henk (1991-03-29).Volatile Compounds in Foods and Beverages.CRC Press.ISBN978-0-8247-8390-7.
- ^Panten, Johannes; Surburg, Horst (2016). "Flavors and Fragrances, 3. Aromatic and Heterocyclic Compounds".Ullmann's Encyclopedia of Industrial Chemistry.pp. 1–45.doi:10.1002/14356007.t11_t02.ISBN978-3-527-30673-2.