Aeruginascin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
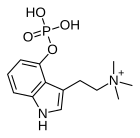
| Formula | C13H20N2O4P |
| Molar mass | 299.287g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
AeruginascinorN,N,N-trimethyl-4-phosphoryloxytryptamineis anindoleaminederivative which occurs naturally within the mushroomsInocybe aeruginascens,[1][2][3][4][5][6]Pholiotina cyanopus,[6]andPsilocybe cubensis.[7]Aeruginascin is theN-trimethylanalogueofpsilocybin.It is closely related to the frog skin toxinbufotenidine(5-HTQ), a potent5-HT3receptoragonist, but the aeruginascin metabolite 4-HO-TMT shows strong binding at the5-HT2 receptorssimilar topsilocin.[8][9]The first scientific literature about the pharmacological effects of aeruginascin is from a study published by Gartz in 1989.[10]Across 23 analyzed cases of accidental hallucinogenic mushroom poisonings, people who had ingested the mushroomInocybe aeruginascensreported only euphoric experiences.[11]This is in contrast to the slight and in some cases extremely dysphoric experiences reported from the accidental ingestion of non-aeruginascin-containing mushrooms (containing solely psilocybin and psilocin).
References[edit]
- ^Gartz J (1995)."Inocybe aeruginascens Babos".Eleusis, Journal of Psychoactive Plants & Compounds.3.Museo Civico di Rovereto: 31–4.
- ^Jensen N, Gartz J, Laatsch H (June 2006)."Aeruginascin, a trimethylammonium analogue of psilocybin from the hallucinogenic mushroom Inocybe aeruginascens"(PDF).Planta Medica.72(7): 665–666.doi:10.1055/s-2006-931576.PMID16673333.S2CID260281286.Archived fromthe original(PDF)on 2011-05-24.
- ^Sherwood AM, Halberstadt AL, Klein AK, McCorvy JD, Kaylo KW, Kargbo RB, Meisenheimer P (February 2020). "Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin".Journal of Natural Products.83(2): 461–467.doi:10.1021/acs.jnatprod.9b01061.PMID32077284.S2CID211214973.
- ^Servillo L, Giovane A, Balestrieri ML, Cautela D, Castaldo D (September 2012). "N-methylated tryptamine derivatives in citrus genus plants: identification of N,N,N-trimethyltryptamine in bergamot".Journal of Agricultural and Food Chemistry.60(37): 9512–9518.doi:10.1021/jf302767e.PMID22957740.
- ^de Carvalho Junior AR, Oliveira Ferreira R, de Souza Passos M, da Silva Boeno SI, Glória das Virgens LL, Ventura TL, et al. (March 2019)."Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Triterpenes and Alkaloids fromPsychotria nuda(Cham. & Schltdl.) Wawra ".Molecules.24(6): 1026.doi:10.3390/molecules24061026.PMC6471101.PMID30875889.
- ^abGotvaldová K, Borovička J, Hájková K, Cihlářová P, Rockefeller A, Kuchař M (November 2022)."Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids".International Journal of Molecular Sciences.23(22): 14068.doi:10.3390/ijms232214068.PMC9693126.PMID36430546.
- ^"CaaMTech Publishes Fundamental Research on Aeruginascin Derivatives".14 September 2022.
- ^Chadeayne AR, Pham DN, Reid BG, Golen JA, Manke DR (July 2020)."Active Metabolite of Aeruginascin (4-Hydroxy-N,N,N-trimethyltryptamine): Synthesis, Structure, and Serotonergic Binding Affinity".ACS Omega.5(27): 16940–16943.doi:10.1021/acs Omega.0c02208.PMC7365549.PMID32685863.
- ^Bauer BE (2020-07-07)."Study Finds Aeruginascin Metabolite 4-HO-TMT is Active at the Serotonin 5-HT2A Receptor".Psychedelic Science Review.Archivedfrom the original on 2020-08-05.Retrieved2021-09-07.
- ^Gartz J (January 1989)."Analysis of Aeruginascin in Fruit Bodies of the Mushroom Inocybe aeruginascens".International Journal of Crude Drug Research.27(3): 141–144.doi:10.3109/13880208909053954.ISSN0167-7314.
- ^"Aeruginascin".Psychedelic Science Review.2018-11-19.Retrieved2021-09-07.

