Alkyne

Inorganic chemistry,analkyneis anunsaturatedhydrocarboncontaining at least onecarbon—carbontriple bond.[1]The simplestacyclicalkynes with only one triple bond and no otherfunctional groupsform ahomologous serieswith the general chemical formulaCnH2n−2.Alkynes are traditionally known as acetylenes, although the nameacetylenealso refers specifically toC2H2,known formally asethyneusingIUPAC nomenclature.Like other hydrocarbons, alkynes are generallyhydrophobic.[2]
Structure and bonding
[edit]In acetylene, the H–C≡Cbond anglesare 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare.Benzynecannot be isolated. The C≡C bond distance of 118picometers(for C2H2) is much shorter than the C=C distance inalkenes(132 pm, for C2H4) or the C–C bond in alkanes (153 pm).[3]

Illustrative alkynes:a,acetylene,b,two depictions of propyne,c,1-butyne,d,2-butyne,e,the naturally occurring 1-phenylhepta-1,3,5-triyne, andf,the strained cycloheptyne. Triple bonds are highlightedblue.
Thetriple bondis very strong with abond strengthof 839 kJ/mol. Thesigma bondcontributes 369 kJ/mol, the firstpi bondcontributes 268 kJ/mol. and the second pi bond 202 kJ/mol. Bonding is usually discussed in the context ofmolecular orbital theory,which recognizes the triple bond as arising from overlap of s and p orbitals. In the language ofvalence bond theory,the carbon atoms in an alkyne bond aresp hybridized:they each have two unhybridizedp orbitalsand twosp hybrid orbitals.Overlap of an sp orbital from each atom forms one sp–spsigma bond.Each p orbital on one atom overlaps one on the other atom, forming two pi bonds, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent acetylene. The two sp orbitals project on opposite sides of the carbon atom.
Terminal and internal alkynes
[edit]Internal alkynes feature carbon substituents on each acetylenic carbon. Symmetrical examples includediphenylacetyleneand3-hexyne.They may also be asymmetrical, such as in2-pentyne.
Terminal alkynes have the formulaRC2H.An example ismethylacetylene(propyne using IUPAC nomenclature). They are often prepared by alkylation ofmonosodium acetylide.[4]Terminal alkynes, likeacetyleneitself, are mildly acidic, with pKavalues of around 25. They are far more acidic than alkenes and alkanes, which have pKavalues of around 40 and 50, respectively. The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo-, silyl-, and alkoxoalkynes. Thecarbanionsgenerated by deprotonation of terminal alkynes are calledacetylides.[5]
Naming alkynes
[edit]Insystematic chemical nomenclature,alkynes are named with the Greek prefix system without any additional letters. Examples include ethyne or octyne. In parent chains with four or more carbons, it is necessary to say where the triple bond is located. Foroctyne,one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. The lowest number possible is given to thetriple bond.When no superior functional groups are present, the parent chain must include the triple bond even if it is not the longest possible carbon chain in the molecule. Ethyne is commonly called by its trivial name acetylene.
In chemistry, thesuffix-yneis used to denote the presence of a triple bond. Inorganic chemistry,the suffix often followsIUPAC nomenclature.However,inorganic compoundsfeaturingunsaturationin the form of triple bonds may be denoted by substitutive nomenclature with the same methods used with alkynes (i.e. the name of the corresponding saturated compound is modified by replacing the "-ane"ending with" -yne ")."-diyne"is used when there are two triple bonds, and so on. The position of unsaturation is indicated by a numericallocantimmediately preceding the "-yne" suffix, or 'locants' in the case of multiple triple bonds. Locants are chosen so that the numbers are low as possible. "-yne" is also used as a suffix to name substituent groups that are triply bound to the parent compound.
Sometimes a number betweenhyphensis inserted before it to state which atoms the triple bond is between. This suffix arose as a collapsed form of the end of the word "acetylene".The final" -e "disappears if it is followed by another suffix that starts with a vowel.[6]
Structural isomerism
[edit]Alkynes having four or morecarbonatoms can form differentstructural isomersby having the triple bond in different positions or having some of the carbon atoms be substituents rather than part of the parent chain. Other non-alkyne structural isomers are also possible.
- C2H2:acetyleneonly
- C3H4:propyneonly
- C4H6:2 isomers:1-butyne,and2-butyne
- C5H8:3 isomers:1-pentyne,2-pentyne,and3-methyl-1-butyne
- C6H10:7 isomers:1-hexyne,2-hexyne,3-hexyne,4-methyl-1-pentyne,4-methyl-2-pentyne,3-methyl-1-pentyne,3,3-dimethyl-1-butyne
Synthesis
[edit]Cracking
[edit]Commercially, the dominant alkyne is acetylene itself, which is used as a fuel and a precursor to other compounds, e.g.,acrylates.Hundreds of millions of kilograms are produced annually bypartial oxidationofnatural gas:[7]
Propyne, also industrially useful, is also prepared bythermal crackingof hydrocarbons.
Dehydrohalogenation and related reactions
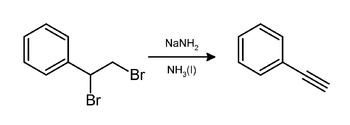
[edit]Alkynes are prepared from 1,1- and 1,2-dihaloalkanesby doubledehydrohalogenation.The reaction provides a means to generate alkynes from alkenes, which are firsthalogenatedand then dehydrohalogenated. For example,phenylacetylenecan be generated fromstyrenebybrominationfollowed by treatment of the resulting ofstyrene dibromidewithsodium amideinammonia:[8][9]
Via theFritsch–Buttenberg–Wiechell rearrangement,alkynes are prepared fromvinyl bromides.Alkynes can be prepared fromaldehydesusing theCorey–Fuchs reactionand from aldehydes orketonesby theSeyferth–Gilbert homologation.
Vinyl halidesare susceptible to dehydrohalogenation.
Reactions, including applications
[edit]Featuring a reactivefunctional group,alkynes participate in manyorganic reactions.Such use was pioneered byRalph Raphael,who in 1955 wrote the first book describing their versatility as intermediates insynthesis.[10]
Hydrogenation
[edit]Being moreunsaturatedthan alkenes, alkynes characteristically undergo reactions that show that they are "doubly unsaturated". Alkynes are capable of adding two equivalents ofH2,whereas an alkene adds only one equivalent.[11]Depending on catalysts and conditions, alkynes add one or two equivalents of hydrogen. Partialhydrogenation,stopping after the addition of only one equivalent to give thealkene,is usually more desirable since alkanes are less useful:

The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of apalladium/silvercatalyst). For more complex alkynes, theLindlar catalystis widely recommended to avoid formation of the alkane, for example in the conversion ofphenylacetylenetostyrene.[12]Similarly,halogenationof alkynes gives the alkene dihalides or alkyl tetrahalides:
The addition of one equivalent ofH2to internal alkynes gives cis-alkenes.
Addition of halogens and related reagents
[edit]Alkynes characteristically are capable of adding two equivalents ofhalogensand hydrogen halides.
The addition of nonpolarE−Hbonds acrossC≡Cis general for silanes, boranes, and related hydrides. Thehydroborationof alkynes gives vinylic boranes which oxidize to the correspondingaldehydeor ketone. In thethiol-yne reactionthe substrate is a thiol.
Addition of hydrogen halides has long been of interest. In the presence ofmercuric chlorideas acatalyst,acetylene andhydrogen chloridereact to givevinyl chloride.While this method has been abandoned in the West, it remains the main production method in China.[13]
Hydration
[edit]Thehydration reactionof acetylene givesacetaldehyde.The reaction proceeds by formation ofvinyl alcohol,whichtautomerizesto form the aldehyde. This reaction was once a major industrial process but it has been displaced by theWacker process.This reaction occurs in nature, the catalyst beingacetylene hydratase.
Hydration ofphenylacetylenegivesacetophenone:[14]
(Ph3P)AuCH3catalyzes hydration of 1,8-nonadiyne to 2,8-nonanedione:[15]
Tautomerism
[edit]Terminal alkyl alkynes exhibit tautomerism.Propyneexists in equilibrium withpropadiene:
Cycloadditions and oxidation
[edit]Alkynes undergo diversecycloadditionreactions. TheDiels–Alder reactionwith 1,3-dienesgives1,4-cyclohexadienes.This general reaction has been extensively developed. Electrophilic alkynes are especially effectivedienophiles.The "cycloadduct" derived from the addition of alkynes to2-pyroneeliminatescarbon dioxideto give thearomaticcompound. Other specialized cycloadditions include multicomponent reactions such asalkyne trimerisationto givearomaticcompounds and the [2+2+1]-cycloaddition of an alkyne,alkeneandcarbon monoxidein thePauson–Khand reaction.Non-carbon reagents also undergo cyclization, e.g.azide alkyne Huisgen cycloadditionto givetriazoles.Cycloaddition processes involving alkynes are often catalyzed by metals, e.g.enyne metathesisandalkyne metathesis,which allows the scrambling ofcarbyne(RC) centers:
Oxidative cleavage of alkynes proceeds via cycloaddition to metal oxides. Most famously,potassium permanganateconverts alkynes to a pair ofcarboxylic acids.
Reactions specific for terminal alkynes
[edit]Terminal alkynes are readily converted to many derivatives, e.g. by coupling reactions and condensations. Via the condensation with formaldehyde and acetylene is producedbutynediol:[7][16]
In theSonogashira reaction,terminal alkynes are coupled with aryl or vinyl halides:
This reactivity exploits the fact that terminal alkynes are weak acids, whose typicalpKavalues around 25 place them between that ofammonia(35) andethanol(16):
The reactions of alkynes with certain metal cations, e.g.Ag+andCu+also gives acetylides. Thus, few drops ofdiamminesilver(I) hydroxide(Ag(NH3)2OH) reacts with terminal alkynes signaled by formation of a white precipitate of the silver acetylide. This reactivity is the basis of alkynecoupling reactions,including theCadiot–Chodkiewicz coupling,Glaser coupling,and theEglinton couplingshown below:[17]
In theFavorskii reactionand inalkynylationsin general, terminal alkynes add tocarbonylcompounds to give thehydroxyalkyne.
Metal complexes
[edit]Alkynes form complexes with transition metals. Such complexes occur also in metal catalyzed reactions of alkynes such asalkyne trimerization.Terminal alkynes, including acetylene itself, react with water to give aldehydes. The transformation typically requires metal catalysts to give this anti-Markovnikov addition result.[18]
Alkynes in nature and medicine
[edit]According toFerdinand Bohlmann,the first naturally occurring acetylenic compound, dehydromatricaria ester, was isolated from anArtemisiaspecies in 1826. In the nearly two centuries that have followed, well over a thousand naturally occurring acetylenes have been discovered and reported.Polyynes,a subset of this class of natural products, have been isolated from a wide variety of plant species, cultures of higher fungi, bacteria, marine sponges, and corals.[19]Some acids liketariric acidcontain an alkyne group. Diynes and triynes, species with the linkage RC≡C–C≡CR′ and RC≡C–C≡C–C≡CR′ respectively, occur in certain plants (Ichthyothere,Chrysanthemum,Cicuta,Oenantheand other members of theAsteraceaeandApiaceaefamilies). Some examples arecicutoxin,oenanthotoxin,andfalcarinol.These compounds are highly bioactive, e.g. asnematocides.[20]1-Phenylhepta-1,3,5-triyne is illustrative of a naturally occurring triyne.
Alkynes occur in some pharmaceuticals, including the contraceptivenoretynodrel.A carbon–carbon triple bond is also present in marketed drugs such as the antiretroviralEfavirenzand the antifungalTerbinafine.Molecules called ene-diynes feature a ring containing an alkene ( "ene" ) between two alkyne groups ( "diyne" ). These compounds, e.g.calicheamicin,are some of the most aggressive antitumor drugs known, so much so that the ene-diyne subunit is sometimes referred to as a "warhead". Ene-diynes undergo rearrangement via theBergman cyclization,generating highly reactive radical intermediates that attack DNA within the tumor.[21]
See also
[edit]References
[edit]- ^Alkyne.Encyclopædia Britannica
- ^Saul Patai, ed. (1978).The Carbon–Carbon Triple Bond.Vol. 1. John Wiley & Sons.ISBN9780470771563.
- ^Smith, Michael B.; March, Jerry (2006).March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure.p. 24.doi:10.1002/0470084960.ISBN9780470084960.
- ^K. N. Campbell, B. K. Campbell (1950). "n-Butylacetylene ".Organic Syntheses.30:15.doi:10.15227/orgsyn.030.0015.
- ^Bloch, Daniel R. (2012).Organic Chemistry Demystified(2nd ed.). McGraw-Hill. p. 57.ISBN978-0-07-176797-2.
- ^The Commission on the Nomenclature of Organic Chemistry (1971) [1958 (A: Hydrocarbons, and B: Fundamental Heterocyclic Systems), 1965 (C: Characteristic Groups)].Nomenclature of Organic Chemistry(3rd ed.). London: Butterworths.ISBN0-408-70144-7.
- ^abGräfje, Heinz; Körnig, Wolfgang; Weitz, Hans-Martin; Reiß, Wolfgang; Steffan, Guido; Diehl, Herbert; Bosche, Horst; Schneider, Kurt; Kieczka, Heinz (2000). "Butanediols, Butenediol, and Butynediol".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a04_455.ISBN978-3527306732.
- ^Kenneth N. Campbell, Barbara K. Campbell (1950). "Phenylacetylene".Organic Syntheses.30:72.doi:10.15227/orgsyn.030.0072.
- ^A. Le Coq and A. Gorgues (1979). "Alkyness via Phase Transfer-Catalyzed Dehydrohalogenatiion: Propiolaldehyde Diethyl Acetal".Organic Syntheses.59:10.doi:10.15227/orgsyn.059.0010.
- ^Raphael, Ralph Alexander (1955).Acetylenic compounds in organic synthesis.London: Butterworths Scientific Publications.OCLC3134811.
- ^Rosser & Williams (1977).Modern Organic Chemistry for A-level.Great Britain: Collins. p. 82.ISBN0003277402.
- ^H. Lindlar; R. Dubuis (1973)."Palladium catalyst for partial reduction of acetylenes".Organic Syntheses;Collected Volumes,vol. 5, p. 880..
- ^Dreher, Eberhard-Ludwig; Torkelson, Theodore R.; Beutel, Klaus K. (2011). "Chlorethanes and Chloroethylenes".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.o06_o01.ISBN978-3527306732.
- ^Fukuda, Y.; Utimoto, K. (1991). "Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst".J. Org. Chem.56(11): 3729.doi:10.1021/jo00011a058.
- ^Mizushima, E.; Cui, D.-M.; Nath, D. C. D.; Hayashi, T.; Tanaka, M. (2005)."Au(I)-Catalyzed hydratation of alkynes: 2,8-nonanedione".Organic Syntheses.83:55.
- ^Peter Pässler; Werner Hefner; Klaus Buckl; Helmut Meinass; Andreas Meiswinkel; Hans-Jürgen Wernicke; Günter Ebersberg; Richard Müller; Jürgen Bässler; Hartmut Behringer; Dieter Mayer (2008). "Acetylene".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a01_097.pub3.ISBN978-3527306732.
- ^K. Stöckel and F. Sondheimer (1974). "[18]Annulene".Organic Syntheses.54:1.doi:10.15227/orgsyn.054.0001.
- ^Hintermann, Lukas; Labonne, Aurélie (2007). "Catalytic Hydration of Alkynes and Its Application in Synthesis".Synthesis.2007(8): 1121–1150.doi:10.1055/s-2007-966002.S2CID95666091.
- ^Annabelle L. K. Shi Shun; Rik R. Tykwinski (2006). "Synthesis of Naturally Occurring Polyynes".Angew. Chem. Int. Ed.45(7): 1034–1057.doi:10.1002/anie.200502071.PMID16447152.
- ^Lam, Jørgen (1988).Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC): proceedings of a Conference on the Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds (NOARC).Amsterdam: Elsevier.ISBN0-444-87115-2.
- ^S. Walker; R. Landovitz; W.D. Ding; G.A. Ellestad; D. Kahne (1992)."Cleavage behavior of calicheamicin gamma 1 and calicheamicin T".Proc Natl Acad Sci USA.89(10): 4608–12.Bibcode:1992PNAS...89.4608W.doi:10.1073/pnas.89.10.4608.PMC49132.PMID1584797.














![{\displaystyle {\ce {2R-\!{\equiv }\!-H->[{\ce {Cu(OAc)2}}][{\ce {pyridine}}]R-\!{\equiv }\!-\!{\equiv }\!-R}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8fe5690544a4da60b118164e70291e6eae02f82e)