Amphibole

Amphibole(/ˈæmfəboʊl/AM-fə-bohl) is a group ofinosilicateminerals,forming prism or needlelike crystals,[1]composed of double chainSiO
4tetrahedra,linked at the vertices and generally containingionsofironand/ormagnesiumin their structures. ItsIMAsymbolis Amp.[2]Amphiboles can be green, black, colorless, white, yellow, blue, or brown. TheInternational Mineralogical Associationcurrently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.[3]
Mineralogy[edit]

Amphiboles crystallize into two crystal systems,monoclinicandorthorhombic.[4]In chemical composition and general characteristics they are similar to thepyroxenes.The chief differences from pyroxenes are that (i) amphiboles contain essential hydroxyl (OH) or halogen (F, Cl) and (ii) the basic structure is a double chain of tetrahedra (as opposed to the single chain structure of pyroxene). Most apparent, in hand specimens, is that amphiboles form oblique cleavage planes (at around 120 degrees), whereas pyroxenes have cleavage angles of approximately 90 degrees. Amphiboles are also specifically less dense than the corresponding pyroxenes.[5]Amphiboles are the primary constituent ofamphibolites.[6]
Structure[edit]
Like pyroxenes, amphiboles are classified as inosilicate (chain silicate) minerals. However, the pyroxene structure is built around single chains of silica tetrahedra while amphiboles are built around double chains of silica tetrahedra. In other words, as with almost all silicate minerals, each silicon ion is surrounded by four oxygen ions. In amphiboles, some of the oxygen ions are shared between silicon ions to form a double chain structure as depicted below. These chains extend along the [001] axis of the crystal. One side of each chain hasapicaloxygen ions, shared by only one silicon ion, and pairs of double chains are bound to each other by metal ions that connect apical oxygen ions. The pairs of double chains have been likened toI-beams.Each I-beam is bonded to its neighbor by additional metal ions to form the complete crystal structure. Large gaps in the structure may be empty or partially filled by large metal ions, such as sodium, but remain points of weakness that help define the cleavage planes of the crystal.[7]
-
Double-chain inosilicate structure looking up the [100] axis. Silicon ions are hidden by apical oxygen ions.
-
Side view (along [010]) of double chain inosilicate backbone. Apical oxygens are at the bottom.
-
Amphibole structure looking along the [001] axis. Silicon ions are emphasized. Two "I-beams" are outlined in green.
In rocks[edit]
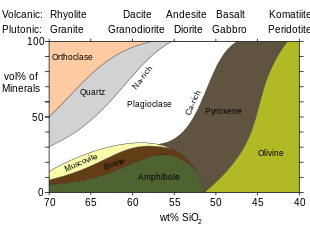


Amphiboles are minerals of eitherigneousormetamorphicorigin. Amphiboles are more common inintermediatetofelsicigneous rocks than inmaficigneous rocks,[8]because the highersilicaand dissolved water content of the moreevolvedmagmas favors formation of amphiboles rather than pyroxenes.[9]The highest amphibole content, around 20%, is found inandesites.[10]Hornblendeis widespread in igneous and metamorphic rocks and is particularly common insyenitesanddiorites.Calcium is sometimes a constituent of naturally occurring amphiboles. Amphilotes of metamorphic origin include those developed inlimestonesby contact metamorphism (tremolite) and those formed by the alteration of other ferromagnesian minerals (such as hornblende as an alteration product of pyroxene).[11]Pseudomorphsof amphibole after pyroxene are known asuralite.[12]
History and etymology[edit]
The nameamphibolederives fromGreekamphíbolos(ἀμφίβολος,lit. 'double entendre'), implying ambiguity. The name was used byRené Just Haüyto include tremolite,actinoliteandhornblende.The group was so named by Haüy in allusion to the protean variety, in composition and appearance, assumed by its minerals. This term has since been applied to the whole group. Numerous sub-species and varieties are distinguished, the more important of which are tabulated below in two series. The formulae of each will be seen to be built on the general double-chainsilicateformula RSi4O11.[13]
Four of the amphibole minerals are commonly calledasbestos.These are: anthophyllite, riebeckite, the cummingtonite/grunerite series, and the actinolite/tremolite series. The cummingtonite/grunerite series is often termedamositeor "brown asbestos", andriebeckiteis known as crocidolite or "blue asbestos". These are generally called amphibole asbestos.[14]Mining, manufacture and prolonged use of these minerals can cause serious illnesses.[15][16]
Mineral species[edit]
The more common amphiboles are classified as shown in the following table:[17]
| Amphibole classification (After Nesse 2000[17]) | |||||||
|---|---|---|---|---|---|---|---|
| Group | W | X2 | Y5 | Z8O22(OH)2 | Mineral | Symmetry | Comment |
| Iron-magnesium | (Mg,Fe)2 | (Mg,Fe)5 | Si8O22(OH)2 | Anthophyllite | Orthorhombic | Orthoamphibolite | |
| (Mg,Fe)2 | (Mg,Fe)3Al2 | Al2Si6O22(OH)2 | Gedrite | ||||
| (Mg,Fe)2 | (Mg,Fe)5 | Si8O22(OH)2 | Cummingtonite-Grunerite | Monoclinic | Low-Ca-clinoamphibolite | ||
| Calcic | Ca2 | (Mg,Fe)5 | Si8O22(OH)2 | Tremolite-actinolite | Ca-clinoamphibole | ||
| (Na,K)0−1 | Ca2 | (Mg,Fe,Fe3+,Al)5 | (Si,Al)8O22(OH)2 | Hornblende | |||
| Na | Ca2 | (Mg,Fe)4Ti | Si6Al2O22(OH)2 | Kaersutite | |||
| Sodic-calcic | Na | NaCa | (Mg,Fe)5 | Si8O22(OH)2 | Richterite | Na-Ca-clinonamphibole | |
| Na | NaCa | (Mg,Fe)4Fe3+ | Si7AlO22(OH)2 | Katophorite | |||
| Sodic | Na2 | (Mg,Fe)3(Al,Fe3+)2 | Si8O22(OH)2 | Glaucophane-riebeckite | Na-clinoamphibole | ||
| Na | Na2 | (Mg,Fe)4(Al,Fe3+) | Si8O22(OH)2 | Eckermanite-arfvedsonite | |||
Other species[edit]
Orthorhombic series
- Holmquistite,Li2Mg3Al2Si8O22(OH)2
Monoclinic series
- Pargasite,NaCa2Mg3Fe2+Si6Al3O22(OH)2
- Winchite,(CaNa)Mg4(Al,Fe3+)Si8O22(OH)2
- Edenite,NaCa2Mg5(Si7Al)O22(OH)2
Series[edit]
Certain amphibole minerals form solid solution series, at least at elevated temperature. Ferrous iron usually substitutes freely for magnesium in amphiboles to form continuous solid solution series between magnesium-rich and iron-rich endmembers. These include the cummington (magnesium) to grunerite (iron) endmembers, where the dividing line is placed at 30% magnesium.[18]
In addition, the orthoamphiboles, anthophyllite and gedrite, which differ in their aluminium content, form a continuous solid solution at elevated temperature. As the amphibole cools, the two end members exsolve to form very thin layers (lamellae).[18]
Hornblende is highly variable in composition, and includes at least five solid solution series: magnesiohornblende-ferrohornblende (Ca2[(Mg,Fe)4Al]Si7AlO22(OH)2), tschermakite-ferrotschermakite (Ca2[(Mg,Fe)3Al2]Si6Al2O22(OH)2), edenite-ferroedenite (NaCa2(Mg,Fe)5Si7AlO22(OH)2), pargasite-ferropargasite (NaCa2[(Mg,Fe)4Al]Si6Al2O22(OH)2) and magnesiohastingstite-hastingsite (NaCa2[(Mg,Fe)4Fe3+]Si67Al2O22(OH)2). In addition, titanium, manganese, or chromium can substitute for some of the cations and oxygen, fluorine, or chlorine for some of the hydroxide. The different chemical types are almost impossible to distinguish even by optical or X-ray methods, and detailed chemical analysis using an electron microprobe is required.[12]
Glaucophane to riebeckite form yet another solid solution series, which also extends towards hornblende and arfvedsonite.[19]
There isnota continuous series between calcic clinoamphiboles, such as hornblende, and low-calcium amphiboles, such as orthoamphiboles or the cummingtonite-grunerite series. Compositions intermediate in calcium are almost nonexistent in nature.[20]However, there is a solid solution series between hornblende and tremolite-actinolite at elevated temperature. Amiscibility gapexists at lower temperatures, and, as a result, hornblende often contains exsolution lamellae of grunerite.[21]
Descriptions[edit]
On account of the wide variations in chemical composition, the different members vary considerably in properties and general appearance.
Anthophylliteoccurs as brownish, fibrous or lamellar masses with hornblende inmica-schistatKongsberginNorwayand some other localities. An aluminous related species is known asgedriteand a deep greenRussianvariety containing littleironaskupfferite.[13]
Hornblendeis an important constituent of many igneous rocks. It is also an important constituent ofamphibolitesformed by metamorphism ofbasalt.[22]
Actinoliteis an important and common member of the monoclinic series, forming radiating groups ofacicularcrystals of a bright green or greyish-green color. It occurs frequently as a constituent ofgreenschists.The name (fromGreekἀκτίς, ἀκτῖνος/aktís, aktînos,a 'ray' andλίθος/líthos,a 'stone') is a translation of the oldGermanwordStrahlstein(radiated stone).[13][23]
Glaucophane,crocidolite,riebeckiteandarfvedsoniteform a somewhat special group of alkali-amphiboles. The first two are blue fibrous minerals, with glaucophane occurring inblueschistsand crocidolite (blue asbestos) in ironstone formations, both resulting from dynamo-metamorphic processes. The latter two are dark green minerals, which occur as original constituents of igneous rocks rich in sodium, such asnepheline-syeniteandphonolite.[13][24]
Pargasiteis a rare magnesium-rich variety of hornblende[12]with essentialsodium,usually found inultramaficrocks. For instance, it occurs in uncommonmantlexenoliths,carried up bykimberlite.It is hard, dense, black and usuallyautomorphic,with a red-brownpleochroisminpetrographicthin section.[25]
See also[edit]
References[edit]
- ^"Amphibole".Dictionary of Geology.Retrieved2013-01-21.
- ^Warr, L.N. (2021)."IMA-CNMNC approved mineral symbols".Mineralogical Magazine.85(3): 291–320.Bibcode:2021MinM...85..291W.doi:10.1180/mgm.2021.43.S2CID235729616.
- ^Mindat, Amphibole Supergroup
- ^Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993).Manual of mineralogy: (after James D. Dana)(21st ed.). New York: Wiley. p. 491.ISBN047157452X.
- ^Klein & Hurlbut 1993,pp. 474–475, 478, 491.
- ^Klein & Hurlbut 1993,pp. 590.
- ^Nesse, William D. (2000).Introduction to mineralogy.New York: Oxford University Press. pp. 277–279.ISBN9780195106916.
- ^Peters, Stefan T. M.; Troll, Valentin R.; Weis, Franz A.; Dallai, Luigi; Chadwick, Jane P.; Schulz, Bernhard (2017-03-16)."Amphibole megacrysts as a probe into the deep plumbing system of Merapi volcano, Central Java, Indonesia".Contributions to Mineralogy and Petrology.172(4): 16.Bibcode:2017CoMP..172...16P.doi:10.1007/s00410-017-1338-0.ISSN1432-0967.S2CID132014026.
- ^Nesse 2000,p. 279–280.
- ^Levin, Harold L. (2010).The earth through time(9th ed.). Hoboken, N.J.: J. Wiley. p. 62.ISBN978-0470387740.
- ^Klein & Hurlbut 1993,p. 496-497.
- ^abcNesse 2000,p. 285.
- ^abcdOne or more of the preceding sentences incorporates text from a publication now in thepublic domain:Spencer, Leonard James(1911). "Amphibole".InChisholm, Hugh(ed.).Encyclopædia Britannica.Vol. 1 (11th ed.). Cambridge University Press. pp. 883–884.
- ^US Geological Survey,Asbestos,accessed 20 July 2015.
- ^Nesse 2000,p. 242.
- ^"Health Effects of Asbestos".Agency for Toxic Substances and Disease Registry.Centers for Disease Control. 10 December 2018.Retrieved6 November2020.
- ^abNesse 2000,p. 278.
- ^abNesse 2000,pp. 277–290.
- ^Nesse 2000,p. 287.
- ^Nesse 2000,p. 279.
- ^Klein & Hurlbut 1993,p. 496.
- ^Nesse 2000,p. 286.
- ^Klein & Hurlbut 1993,pp. 495–496.
- ^Nesse 2000,pp. 287–289.
- ^ "Pargasite"(PDF).Handbook of Mineralogy(pdf).Mineralogical Society of America.Retrieved2012-12-17.

![Double-chain inosilicate structure looking up the [100] axis. Silicon ions are hidden by apical oxygen ions.](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d5/Amphibole_100.jpg/111px-Amphibole_100.jpg)
![Side view (along [010]) of double chain inosilicate backbone. Apical oxygens are at the bottom.](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b7/Amphibole_010.jpg/120px-Amphibole_010.jpg)
![Amphibole structure looking along the [001] axis. Silicon ions are emphasized. Two "I-beams" are outlined in green.](https://upload.wikimedia.org/wikipedia/commons/thumb/a/a8/Amphibole_001.jpg/120px-Amphibole_001.jpg)
