Amrinone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Inocor |
| Other names | inamrinone (USANUS) |
| AHFS/Drugs | International Drug Names |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Bioavailability | n/a |
| Protein binding | 10 to 49% |
| Metabolism | Hepatic |
| Eliminationhalf-life | 5 to 8 hours |
| Excretion | Renal(63%) and fecal (18%) |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.056.700 |
| Chemical and physical data | |
| Formula | C10H9N3O |
| Molar mass | 187.202g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amrinone,also known asinamrinone,and sold asInocor,is apyridinephosphodiesterase 3 inhibitor.[1]It is a drug that may improve the prognosis in patients with congestive heart failure.[2]Amrinone has been shown to increase the contractions initiated in the heart by high-gaincalcium induced calcium release(CICR).[3]The positiveinotropiceffect of amrinone is mediated by the selective enhancement of high-gain CICR, which contributes to the contraction of myocytes by phosphorylation through cAMP dependent protein kinase A (PKA) and Ca2+calmodulin kinasepathways.[3]
Actions[edit]
Increases cardiac contractility, vasodilator. Acts by inhibiting the breakdown of both cAMP and cGMP by the phosphodiesterase (PDE3) enzyme. There is a long-standing controversy regarding whether the drug actually increases cardiac contractility in diseased myocardium (and therefore whether it is of any clinical use). The issue has been reviewed extensively by DrPeter Wilmshurst,one of the first cardiologists and researchers to question the drug's efficacy.[4]
PDE-III inhibition and cardiac function[edit]
PDE III is present in cardiac muscle, vascular smooth muscle and platelets. PDE III degrades the phosphodiester bond in cAMP to break it down.[5][6]When PDE III is inhibited, cAMP cannot be inactivated. An increase in cAMP with the administration of amrinone invascular smooth muscleproduces vasodilation by facilitating calcium uptake by thesarcoplasmic reticulum(a special type ofsmooth ER) and decreasing the calcium available for contraction.[5][7]In myocytes, the increase of cAMP concentration increases in turn the activity ofPKA;thiskinaseimproves the Ca2+inward current through the L-type Ca2+channels, which leads tocalcium-induced calcium releasefrom the sarcoplasmic reticulum, giving rise to acalcium sparkthat triggers the contraction; this results in aninotropiceffect. Furthermore, PKA phosphorylates and deactivates thephospholambansthat inhibitSERCA,which is an enzymatic pump that, to terminate the contraction, removes the Ca2+from the cytoplasm, stores it back in the sarcoplasmic reticulum and promotes the subsequent arterial relaxation as well, producing alusitropiceffect. Both inotropic and lusitropic effects justify the use of amrinone to treatheart failure. Amrinone decreases the pulmonary capillary wedge pressure while increasing cardiac output, as it functions as an arterial vasodilator and increases venous capacitance while decreasing venous return.[5]There is a net decrease in myocardial wall tension, and O2consumption when using amrinone. Amrinone also has beneficial effects duringdiastolein the left ventricle, including relaxation,complianceand filling in patients with congestive heart failure.[5]
Indications[edit]
Short-term management of severe CHF (not used long term because of increased mortality, probably due to heart failure).
Effects in congestive heart failure[edit]
Congestive heart failure (CHF) is characterized by a reduction in ventricular performance and abnormalities in peripheral circulation and organs.[6]A reduced release of endothelium derived rela xing factor (EDRF) causes a decrease in the stimulation of guanylate cyclase, andcyclic GMP(cGMP) levels fall in vascular smooth muscle. This impairs relaxation in the vasculature and is a part of the vicious cycle of CHF.[6]Patients with CHF have a down-regulation of their β-1 adrenergic receptors which alters their ability to activate intracellular adenylate cyclase, which catalyzes cAMP formation.[5]cAMP is the second messenger that controls the level of calcium available to allow the heart to contract. An IV administration of amrinone has been shown to increasecardiac output(CO) andstroke volume(SV), while concurrently reducing the filling pressure of the left ventricle and decreasing the resistance in the peripheral vasculature.[2][8][9]This does not lead to an increase in heart rate or blood pressure.[2][8][9]The improvement in performance of the ventricles is likely to result from a direct stimulation of the depressed myocardium as well as a decrease inperipheral vascular resistance.[10]
Contraindications[edit]
Patients withaortic stenosis,hypertrophic cardiomyopathy,or history of hypersensitivity to the drug.
Precautions[edit]
May increase myocardial ischemia. Blood pressure, pulse, and ECG should be constantly monitored. Amrinone should only be diluted with normal saline or 1/2 normal saline; no dextrose solutions should be used.Furosemide,aloop diuretic,should not be administered into an IV line delivering amrinone.
Side effects[edit]
Thrombocytopeniais the most prominent and dose-related side effect, but it is transient and asymptomatic.Nausea,diarrhea,hepatotoxicity,arrhythmiasand fever are other adverse effects.
Amrinone discovery and progression[edit]
Early studies in patients with heart failure showed that amrinone produced short-term hemodynamic improvement, but had limited long-term clinical benefit.[7]Some serious side effects of long term administration included sustainedventricular tachycardiaresulting in circulatory collapse, worsening myocardial ischemia, acute myocardial infarction, and worsening congestive heart failure.[7][11]Amrinone has good absorption from the gastrointestinal tract[12]and has led to gastrointestinal upset when taken orally. The oral form of the drug is no longer in use.[11]Currently, only acute intravenous administration takes place.[11]The effects of amrinone vary widely with species and experimental condition; therefore, its inotropic effects are variable.[3]A loss in sensitivity tophosphodiesterase 3inhibitors, including amrinone, has been observed in end stage heart failure in humans; other treatment options may be more useful for improvement in these stages.[3]
Naming[edit]
Amrinone is theINN,while inamrinone is theUnited States Adopted Name,which was adopted in 2000 in an attempt to avoid confusion withamiodarone.[13]
Synthesis[edit]
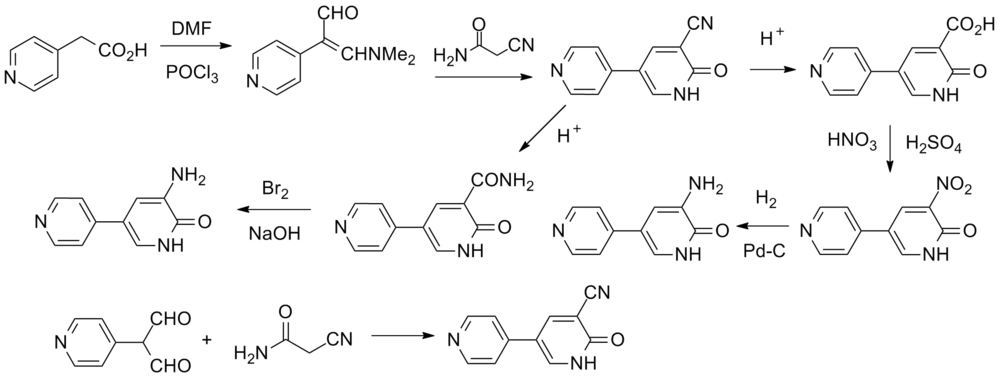
See also:MilrinoneandPelrinone.
References[edit]
- ^Hamada Y, Kawachi K, Yamamoto T, Nakata T, Kashu Y, Sato M, Watanabe Y (August 1999)."Effects of single administration of a phosphodiesterase III inhibitor during cardiopulmonary bypass: comparison of milrinone and amrinone".Japanese Circulation Journal.63(8): 605–609.doi:10.1253/jcj.63.605.PMID10478810.
- ^abcKlein NA, Siskind SJ, Frishman WH, Sonnenblick EH, LeJemtel TH (July 1981). "Hemodynamic comparison of intravenous amrinone and dobutamine in patients with chronic congestive heart failure".The American Journal of Cardiology.48(1): 170–175.doi:10.1016/0002-9149(81)90587-7.PMID7246440.
- ^abcdXiong W, Ferrier GR, Howlett SE (August 2004). "Diminished inotropic response to amrinone in ventricular myocytes from myopathic hamsters is linked to depression of high-gain Ca2+-induced Ca2+ release".The Journal of Pharmacology and Experimental Therapeutics.310(2): 761–773.doi:10.1124/jpet.103.064873.PMID15064331.S2CID6036283.
- ^Wilmshurst P."The HealthWatch Award 2003: Dr Peter Wilmshurst -" Obstacles to honesty in medical research "".Archived fromthe originalon 2015-04-25.
- ^abcdeLevy JH, Ramsay J, Bailey JM (1990). "Pharmacokinetics and pharmacodynamics of phosphodiesterase-III inhibitors".Journal of Cardiothoracic Anesthesia.4(6): 7–11.doi:10.1016/0888-6296(90)90226-6.
- ^abcLeJemtel TH, Scortichini D, Levitt B, Sonnenblick EH (January 1989). "Effects of phosphodiesterase inhibition on skeletal muscle vasculature".The American Journal of Cardiology.63(2): 27A–30A.doi:10.1016/0002-9149(89)90389-5.PMID2909994.
- ^abcPacker M, Medina N, Yushak M (December 1984). "Hemodynamic and clinical limitations of long-term inotropic therapy with amrinone in patients with severe chronic heart failure".Circulation.70(6): 1038–1047.doi:10.1161/01.cir.70.6.1038.PMID6388899.S2CID6325011.
- ^abBenotti JR, Grossman W, Braunwald E, Carabello BA (July 1980)."Effects of amrinone on myocardial energy metabolism and hemodynamics in patients with severe congestive heart failure due to coronary artery disease".Circulation.62(1): 28–34.doi:10.1161/01.cir.62.1.28.PMID7379283.
- ^abKonstam MA, Cohen SR, Weiland DS, Martin TT, Das D, Isner JM, Salem DN (February 1986). "Relative contribution of inotropic and vasodilator effects to amrinone-induced hemodynamic improvement in congestive heart failure".The American Journal of Cardiology.57(4): 242–248.doi:10.1016/0002-9149(86)90899-4.PMID3004184.
- ^LeJemtel TH, Keung E, Ribner HS, Davis R, Wexler J, Blaufox JD, Sonnenblick EH (January 1980). "Sustained beneficial effects of oral amrinone on cardiac and renal function in patients with severe congestive heart failure".The American Journal of Cardiology.45(1): 123–129.doi:10.1016/0002-9149(80)90229-5.PMID7350759.
- ^abcRettig G, Sen S, Fröhlig G, Schieffer H, Bette L (July 1986). "Withdrawal of long-term amrinone therapy in patients with congestive heart failure: a placebo controlled trial".European Heart Journal.7(7): 628–631.doi:10.1093/oxfordjournals.eurheartj.a062114.PMID3530768.
- ^Alousi AA, Farah AE, Lesher GY, Opalka CJ (November 1979)."Cardiotonic activity of amrinone--Win 40680 [5-amino-3,4'-bipyridine-6(1H)-one]".Circulation Research.45(5): 666–677.doi:10.1161/01.RES.45.5.666.PMID39684.
- ^"Amrinone Becomes Inamrinone".USP Quality Review.73.United States Pharmacopeia.March 2000. Archived fromthe originalon 2008-10-03.
- ^US Patent 4004012,Lesher GY, Opalka CJ, "3-Cyano-5-(pyridinyl)-2(1H)-pyridinones", issued 18 January 1977, assigned to STWB Inc.
- ^US Patent 4072746,Lesher GY, Opalka CJ, "3-Amino-5-(pyridinyl)-2(1H)-pyridinones", issued 7 February 1978, assigned to Aventis Pharmaceuticals Inc.
- ^US 4107315,Lesher GY, Opalka CJ, "5-(Pyridinyl)-2(1H)-pyridinones", issued 15 August 1978, assigned to Aventis Pharmaceuticals Inc.
- ^GB 2070008,Gelotte KO, Parady ED, "Process for preparing 5-cyano(3,4'-bipyridin)-6(1H)-one", published 3 September 1981, issued 18 April 1984, assigned toSterling Drum Inc.
