Anti-predator adaptation

Anti-predator adaptationsare mechanisms developed throughevolutionthat assistpreyorganisms in their constant struggle againstpredators.Throughout the animal kingdom, adaptations have evolved for every stage of this struggle, namely by avoiding detection, warding off attack, fighting back, or escaping when caught.
The first line of defence consists in avoiding detection, through mechanisms such ascamouflage,masquerade,apostatic selection,living underground, ornocturnality.
Alternatively, prey animals may ward off attack, whether by advertising the presence of strong defences inaposematism,bymimickinganimals which do possess such defences, bystartlingthe attacker, bysignalling to the predatorthat pursuit is not worthwhile, bydistraction,by using defensive structures such as spines, and byliving in a group.Members of groups are atreduced risk of predation,despite the increased conspicuousness of a group, through improved vigilance, predator confusion, and the likelihood that the predator will attack some other individual.
Avoiding detection[edit]
Staying out of sight[edit]

Animals may avoid becoming prey by living out of sight of predators, whetherin caves,burrows,or by beingnocturnal.[2][3][4][5]Nocturnality is an animal behavior characterized by activity during the night and sleeping during the day. This is a behavioral form of detection avoidance calledcrypsisused by animals to either avoid predation or to enhance prey hunting. Predation risk has long been recognized as critical in shaping behavioral decisions. For example, this predation risk is of prime importance in determining the time of evening emergence in echolocatingbats.Although early access during brighter times permits easier foraging, it also leads to a higher predation risk frombat hawksandbat falcons.This results in an optimum evening emergence time that is a compromise between the conflicting demands.[4]Another nocturnal adaptation can be seen inkangaroo rats.They forage in relatively open habitats, and reduce their activity outside their nest burrows in response to moonlight. During a full moon, they shift their activity towards areas of relatively dense cover to compensate for the extra brightness.[5]

Camouflage[edit]
Camouflageuses any combination of materials, coloration, or illumination for concealment to make the organism hard to detect by sight. It is common in both terrestrial and marine animals. Camouflage can be achieved in many different ways, such as through resemblance to surroundings,disruptive coloration,shadow elimination bycountershadingorcounter-illumination,self-decoration, cryptic behavior, or changeable skin patterns and colour.[6][7]Animals such as theflat-tail horned lizardof North America have evolved to eliminate their shadow and blend in with the ground. The bodies of these lizards are flattened, and their sides thin towards the edge. This body form, along with the white scales fringed along their sides, allows the lizards to effectively hide their shadows. In addition, these lizards hide any remaining shadows by pressing their bodies to the ground.[2]
Masquerade[edit]

Animals can hide in plain sight bymasqueradingas inedible objects. For example, thepotoo,a South American bird, habitually perches on a tree, convincingly resembling a broken stump of a branch,[8]while a butterfly,Kallima,looks just like a dead leaf.[9]
Apostatic selection[edit]
Another way to remain unattacked in plain sight is to look different from other members of the same species. Predators such astitsselectively hunt for abundant types of insect, ignoring less common types that were present, formingsearch imagesof the desired prey. This creates a mechanism for negativefrequency-dependent selection,apostatic selection.[10]
Warding off attack[edit]

Many species make use of behavioral strategies to deter predators.[11]
Startling the predator[edit]
Many weakly-defended animals, includingmoths,butterflies,mantises,phasmids,andcephalopodssuch as octopuses, make use of patterns of threatening orstartling behaviour,such as suddenly displaying conspicuouseyespots,so as to scare off or momentarily distract a predator, thus giving the prey animal an opportunity to escape. In the absence of toxins or other defences, this is essentially bluffing, in contrast to aposematism which involves honest signals.[12][13][14]
Pursuit-deterrent signals[edit]

Pursuit-deterrent signals are behavioral signals used by prey to convince predators not to pursue them. For example,gazellesstot,jumping high with stiff legs and an arched back. This is thought to signal to predators that they have a high level of fitness and can outrun the predator. As a result, predators may choose to pursue a different prey that is less likely to outrun them.[15] White-tailed deerand other prey mammals flag with conspicuous (often black and white) tail markings when alarmed, informing the predator that it has been detected.[16] Warning calls given by birds such as theEurasian jayare similarlyhonest signals,benefiting both predator and prey: the predator is informed that it has been detected and might as well save time and energy by giving up the chase, while the prey is protected from attack.[17][18]
Playing dead[edit]

Another pursuit-deterrent signal isthanatosis or playing dead.Thanatosis is a form of bluff in which an animal mimics its own dead body,feigningdeath to avoid being attacked by predators seeking live prey. Thanatosis can also be used by the predator in order to lure prey into approaching.[19]
An example of this is seen inwhite-tailed deerfawns, which experience a drop in heart rate in response to approaching predators. This response, referred to as "alarmbradycardia",causes the fawn's heart rate to drop from 155 to 38 beats per minute within one beat of the heart. This drop in heart rate can last up to two minutes, causing the fawn to experience a depressed breathing rate and decrease in movement, called tonic immobility. Tonic immobility is a reflex response that causes the fawn to enter a low body position that simulates the position of a corpse. Upon discovery of the fawn, the predator loses interest in the" dead "prey. Other symptoms of alarm bradycardia, such as salivation, urination, and defecation, can also cause the predator to lose interest.[20]
Distraction[edit]

Marinemolluscssuch assea hares,cuttlefish,squidandoctopusesgive themselves a last chance to escape by distracting their attackers. To do this, they eject a mixture of chemicals, which maymimic foodor otherwise confuse predators.[21][22]In response to a predator, animals in these groups releaseink,creating a cloud, and opaline, affecting the predator's feeding senses, causing it to attack the cloud.[21][23]
Distraction displaysattract the attention of predators away from an object, typically the nest or young, that is being protected,[24]as when some birds feign a broken wing while hopping about on the ground.[25]
Mimicry and aposematism[edit]

Mimicryoccurs when an organism (the mimic) simulates signal properties of another organism (the model) to confuse a third organism. This results in the mimic gaining protection, food, and mating advantages.[26]There are two classical types of defensive mimicry: Batesian and Müllerian. Both involveaposematiccoloration,or warning signals, to avoid being attacked by a predator.[27][28]
InBatesian mimicry,a palatable, harmless prey species mimics the appearance of another species that is noxious to predators, thus reducing the mimic's risk of attack.[27]This form of mimicry is seen in manyinsects.The idea behind Batesian mimicry is that predators that have tried to eat the unpalatable species learn to associate its colors and markings with an unpleasant taste. This results in the predator learning to avoid species displaying similar colours and markings, including Batesian mimics, which are in effect parasitic on the chemical or other defences of the unprofitable models.[29][30]Some species of octopus can mimic a selection of other animals by changing their skin color, skin pattern and body motion. When a damselfish attacks an octopus, the octopus mimics a banded sea-snake.[31]The model chosen varies with the octopus's predator and habitat.[32]Most of these octopuses use Batesian mimicry, selecting an organism repulsive to predators as a model.[33][34]
InMüllerian mimicry,two or more aposematic forms share the same warning signals,[27][35]as inviceroyandmonarch butterflies.Birds avoid eating both species because their wing patterns honestly signal their unpleasant taste.[28]

Defensive structures[edit]
Many animals are protected against predators with armour in the form of hard shells (such as mostmolluscsandturtles), leathery or scaly skin (as inreptiles), or tough chitinous exoskeletons (as inarthropods).[25]
Aspineis a sharp, needle-like structure used to inflict pain on predators. An example of this seen in nature is in thesohal surgeonfish.These fish have a sharp scalpel-like spine on the front of each of their tail fins, able to inflict deep wounds. The area around the spines is often brightly colored to advertise the defensive capability;[36]predators often avoid the Sohal surgeonfish.[37]Defensive spines may be detachable, barbed or poisonous.Porcupinespines are long, stiff, break at the tip, and in some species are barbed to stick into a would-be predator. In contrast, thehedgehog's short spines, which are modified hairs,[38]readily bend, and are barbed into the body, so they are not easily lost; they may be jabbed at an attacker.[37]

Many species of slug caterpillar,Limacodidae,have numerous protuberances and stinging spines along their dorsal surfaces. Species that possess these stinging spines suffer less predation than larvae that lack them, and a predator, thepaper wasp,chooses larvae without spines when given a choice.[39]
Safety in numbers[edit]
Group living can decrease the risk of predation to the individual in a variety of ways,[40]as described below.
Dilution effect[edit]
A dilution effect is seen when animals living in a group "dilute" their risk of attack, each individual being just one of many in the group.George C. WilliamsandW.D. Hamiltonproposed that group living evolved because it provides benefits to the individual rather than to the group as a whole, which becomes more conspicuous as it becomes larger. One common example is theshoalingof fish. Experiments provide direct evidence for the decrease in individual attack rate seen with group living, for example inCamargue horsesin Southern France. Thehorse-flyoften attacks these horses, sucking blood and carrying diseases. When the flies are most numerous, the horses gather in large groups, and individuals are indeed attacked less frequently.[41]Water stridersare insects that live on the surface of fresh water, and are attacked from beneath by predatory fish. Experiments varying the group size of the water striders showed that the attack rate per individual water strider decreases as group size increases.[42]
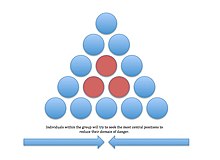
Selfish herd[edit]
The selfish herd theory was proposed byW.D. Hamiltonto explain why animals seek central positions in a group.[43]The theory's central idea is to reduce the individual's domain of danger. A domain of danger is the area within the group in which the individual is more likely to be attacked by a predator. The center of the group has the lowest domain of danger, so animals are predicted to strive constantly to gain this position. Testing Hamilton's selfish herd effect, Alta De Vos and Justin O'Rainn (2010) studiedbrown fur sealpredation fromgreat white sharks.Using decoy seals, the researchers varied the distance between the decoys to produce different domains of danger. The seals with a greater domain of danger had an increased risk of shark attack.[44]
Predator satiation[edit]

A radical strategy for avoiding predators which may otherwise kill a large majority of the emerging stage of a population is to emerge very rarely, at irregular intervals. Predators with a life-cycle of one or a few years are unable to reproduce rapidly enough in response to such an emergence. Predators may feast on the emerging population, but are unable to consume more than a fraction of the brief surfeit of prey.Periodical cicadas,which emerge at intervals of 13 or 17 years, are often used as an example of thispredator satiation,though other explanations of their unusual life-cycle have been proposed.[45]

Alarm calls[edit]
Animals that live in groups often givealarm callsthat give warning of an attack. For example,vervet monkeysgive different calls depending on the nature of the attack: for aneagle,a disyllabic cough; for aleopardor other cat, a loud bark; for aPythonor other snake, a "chutter". The monkeys hearing these calls respond defensively, but differently in each case: to the eagle call, they look up and run into cover; to the leopard call, they run up into the trees; to the snake call, they stand on two legs and look around for snakes, and on seeing the snake, they sometimes mob it. Similar calls are found in other species of monkey, while birds also give different calls that elicit different responses.[46]
Improved vigilance[edit]

In the improved vigilance effect, groups are able to detect predators sooner than solitary individuals.[47]For many predators, success depends on surprise. If the prey is alerted early in an attack, they have an improved chance of escape. For example,wood pigeonflocks are preyed upon bygoshawks.Goshawks are less successful when attacking larger flocks of wood pigeons than they are when attacking smaller flocks. This is because the larger the flock size, the more likely it is that one bird will notice the hawk sooner and fly away. Once one pigeon flies off in alarm, the rest of the pigeons follow.[48]Wildostrichesin Tsavo National Park in Kenya feed either alone or in groups of up to four birds. They are subject to predation by lions. As the ostrich group size increases, the frequency at which each individual raises its head to look for predators decreases. Because ostriches are able to run at speeds that exceed those of lions for great distances, lions try to attack an ostrich when its head is down. By grouping, the ostriches present the lions with greater difficulty in determining how long the ostriches' heads stay down. Thus, although individual vigilance decreases, the overall vigilance of the group increases.[49]

Predator confusion[edit]
Individuals living in large groups may be safer from attack because the predator may be confused by the large group size. As the group moves, the predator has greater difficulty targeting an individual prey animal. Thezebrahas been suggested by the zoologistMartin Stevensand his colleagues as an example of this. When stationary, a single zebra stands out because of its large size. To reduce the risk of attack, zebras often travel in herds. The striped patterns of all the zebras in the herd may confuse the predator, making it harder for the predator to focus in on an individual zebra. Furthermore, when moving rapidly, the zebra stripes create a confusing, flickeringmotion dazzleeffect in the eye of the predator.[50]
Fighting back[edit]
Defensive structures such as spines may be used both to ward off attack as already mentioned, and if need be to fight back against a predator.[37]Methods of fighting back include chemical defences,[51]mobbing,[52]defensive regurgitation,[53]and suicidal altruism.[54]
Chemical defences[edit]

Many prey animals, and to defend againstseed predationalso seeds of plants,[55]make use of poisonous chemicals for self-defence.[51][56]These may be concentrated in surface structures such as spines or glands, giving an attacker a taste of the chemicals before it actually bites or swallows the prey animal: many toxins are bitter-tasting.[51]A last-ditch defence is for the animal's flesh itself to be toxic, as in thepuffer fish,danaid butterfliesandburnet moths.Many insects acquire toxins from their food plants;Danauscaterpillars accumulate toxiccardenolidesfrom milkweeds (Asclepiadaceae).[56]
Some prey animals are able to eject noxious materials to deter predators actively. Thebombardier beetlehas specialized glands on the tip of its abdomen that allows it to direct a toxic spray towards predators. The spray is generated explosively through oxidation of hydroquinones and is sprayed at a temperature of 100 °C.[57]Armoured cricketssimilarly release blood at their joints when threatened (autohaemorrhaging).[58]Several species ofgrasshopperincludingPoecilocerus pictus,[59]Parasanaa donovani,[59]Aularches miliaris,[59]andTegra novaehollandiaesecrete noxious liquids when threatened, sometimes ejecting these forcefully.[59]Spitting cobrasaccurately squirt venom from their fangs at the eyes of potential predators,[60]striking their target eight times out of ten, and causing severe pain.[61]Termite soldiers in theNasutitermitinaehave afontanellar gun,a gland on the front of their head which can secrete and shoot an accurate jet of resinousterpenes"many centimeters". The material is sticky and toxic to other insects. One of the terpenes in the secretion,pinene,functions as analarm pheromone.[62]Seeds deter predation with combinations of toxic non-proteinamino acids,cyanogenic glycosides,proteaseand amylase inhibitors, andphytohemagglutinins.[55]
A few vertebrate species such as theTexas horned lizardare able to shoot squirts of blood from their eyes, by rapidly increasing the blood pressure within the eye sockets, if threatened. Because an individual may lose up to 53% of blood in a single squirt,[63]this is only used against persistent predators like foxes, wolves and coyotes (Canidae), as a last defence.[64]Canids often drop horned lizards after being squirted, and attempt to wipe or shake the blood out of their mouths, suggesting that the fluid has a foul taste;[65]they choose other lizards if given the choice,[66]suggesting a learned aversion towards horned lizards as prey.[66]
The slime glands along the body of thehagfishsecrete enormous amounts of mucus when it is provoked or stressed. The gelatinous slime has dramatic effects on the flow and viscosity of water, rapidly clogging the gills of any fish that attempt to capture hagfish; predators typically release the hagfish within seconds. Common predators of hagfish include seabirds, pinnipeds and cetaceans, but few fish, suggesting that predatory fish avoid hagfish as prey.[67]
Communal defence[edit]

In communal defence, prey groups actively defend themselves by grouping together, and sometimes by attacking or mobbing a predator, rather than allowing themselves to be passive victims of predation.Mobbingis the harassing of a predator by many prey animals. Mobbing is usually done to protect the young in social colonies. For example,red colobusmonkeys exhibit mobbing when threatened bychimpanzees,a common predator. The male red colobus monkeys group together and place themselves between predators and the group's females and juveniles. The males jump together and actively bite the chimpanzees.[52]Fieldfaresare birds which may nest either solitarily or in colonies. Within colonies, fieldfares mob and defecate on approaching predators, shown experimentally to reduce predation levels.[68]
Defensive regurgitation[edit]

Some birds and insects use defensive regurgitation to ward off predators. Thenorthern fulmarvomits a bright orange, oily substance calledstomach oilwhen threatened.[53]The stomach oil is made from their aquatic diets. It causes the predator's feathers to mat, leading to the loss of flying ability and the loss of water repellency.[53]This is especially dangerous for aquatic birds because their water repellent feathers protect them fromhypothermiawhen diving for food.[53]
European rollerchicks vomit a bright orange, foul smelling liquid when they sense danger. This repels prospective predators and may alert their parents to danger: they respond by delaying their return.[69]
Numerous insects utilize defensive regurgitation. Theeastern tent caterpillarregurgitates a droplet of digestive fluid to repel attacking ants.[70]Similarly, larvae of thenoctuid mothregurgitate when disturbed by ants. The vomit of noctuid moths has repellent and irritant properties that help to deter predator attacks.[71]
Suicidal altruism[edit]
An unusual type of predator deterrence is observed in theMalaysian exploding ant.Socialhymenopterarely onaltruismto protect the entire colony, so the self-destructive acts benefit all individuals in the colony.[54]When a worker ant's leg is grasped, it suicidally expels the contents of its hypertrophiedsubmandibular glands,[54]expelling corrosive irritant compounds and adhesives onto the predator. These prevent predation and serve as a signal to other enemy ants to stop predation of the rest of the colony.[72]
Escaping[edit]

Flight[edit]
The normal reaction of a prey animal to an attacking predator is to flee by any available means, whether flying, gliding,[73]falling, swimming, running, jumping,burrowing[74]orrolling,[75]according to the animal's capabilities.[76]Escape paths are often erratic, making it difficult for the predator to predict which way the prey will go next: for example, birds such assnipe,ptarmiganandblack-headed gullsevade fast raptors such asperegrine falconswith zigzagging or jinking flight.[76]In the tropical rain forests of Southeast Asia in particular, many vertebrates escape predators by falling and gliding.[73]Among the insects, many moths turn sharply, fall, or perform a powered dive in response to thesonar clicks of bats.[76]Among fish, thesticklebackfollows a zigzagging path, often doubling back erratically, when chased by a fish-eatingmerganserduck.[76]
Autotomy[edit]

Some animals are capable ofautotomy(self-amputation), shedding one of their ownappendagesin a last-ditch attempt to elude a predator's grasp or to distract the predator and thereby allow escape. The lost body part may beregeneratedlater. Certainsea slugsdiscard stinging papillae; arthropods such ascrabscan sacrifice a claw, which can be regrown over several successive moults; amongvertebrates,manygeckosand otherlizardsshed their tails when attacked: the tail goes on writhing for a while, distracting the predator, and giving the lizard time to escape; a smaller tail slowly regrows.[77]
History of observations[edit]
Aristotlerecorded observations (around 350 BC) of the antipredator behaviour ofcephalopodsin hisHistory of Animals,including the use of ink as a distraction, camouflage, and signalling.[78]
In 1940,Hugh Cottwrote a compendious study of camouflage, mimicry, and aposematism,Adaptive Coloration in Animals.[6]
By the 21st century, adaptation to life incitieshad markedly reduced the antipredator responses of animals such as rats and pigeons; similar changes are observed in captive anddomesticatedanimals.[79]
See also[edit]
- Ecology of fear (concept)– Psychological impact induced by predators
- Plant defense against herbivory– Plants' defenses against being eaten
References[edit]
- ^Zintzen, Vincent; Roberts, Clive D.;Anderson, Marti J.;Stewart, Andrew L.; Struthers, Carl D.; Harvey, Euan S. (2011)."Hagfish Slime as a Defense Mechanism against Gill-breathing Predators".Scientific Reports.1:2011.Bibcode:2011NatSR...1E.131Z.doi:10.1038/srep00131.PMC3216612.PMID22355648.
- ^abSherbrooke, W. C. (2003).Introduction to horned lizards of North America.University of California Press. pp. 117–118.
- ^Cott, H.B. (1940).Adaptive Coloration in Animals.London: Methuen. pp.330–335.
- ^abDuverge, P.L.; Jones, G; Rydell, J.; Ransome, R. (2000)."Functional significance of emergence timing in bats".Ecography.23(1): 32–40.Bibcode:2000Ecogr..23...32D.doi:10.1111/j.1600-0587.2000.tb00258.x.
- ^abDaly, M.; Behrends, P.R.; Wilson, M.; Jacobs, L. (1992). "Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami".Animal Behaviour.44:1–9.doi:10.1016/s0003-3472(05)80748-1.S2CID4077513.
- ^abCott 1940.
- ^Caro 2005,pp. 35–60.
- ^Caro 2005,pp. 53–55.
- ^Cott 1940,pp. 318–320.
- ^Caro 2005,pp. 61–65.
- ^Cooper, William E."Antipredatory Behavior".IDEA.University of California, Riverside. Archived fromthe originalon 18 May 2018.Retrieved23 October2014.
- ^Stevens, Martin(2005). "The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera".Biological Reviews.80(4): 573–588.doi:10.1017/S1464793105006810.PMID16221330.S2CID24868603.
- ^Edmunds, Malcolm (2012)."Deimatic Behavior".Springer.Retrieved31 December2012.
- ^Smith, Ian (3 December 2012)."Octopus vulgaris. Dymantic display".The Conchological Society of Great Britain and Ireland.Retrieved1 January2013.
- ^Caro, T. M.(1986). "The functions of stotting in Thomson's gazelles: Some tests of the predictions".Animal Behaviour.34(3): 663–684.doi:10.1016/S0003-3472(86)80052-5.S2CID53155678.
- ^Bildstein, Keith L. (May 1983). "Why White-Tailed Deer Flag Their Tails".The American Naturalist.121(5): 709–715.doi:10.1086/284096.JSTOR2460873.S2CID83504795.
- ^Bergstrom, C. T.; Lachmann, M. (2001). "Alarm calls as costly signals of antipredator vigilance: the watchful babbler game".Animal Behaviour.61(3): 535–543.CiteSeerX10.1.1.28.773.doi:10.1006/anbe.2000.1636.S2CID2295026.
- ^Getty, T. (2002). "The discriminating babbler meets the optimal diet hawk".Anim. Behav.63(2): 397–402.doi:10.1006/anbe.2001.1890.S2CID53164940.
- ^Pasteur, Georges (1982). "A classificatory review of mimicry systems".Annual Review of Ecology and Systematics.13:169–199.doi:10.1146/annurev.es.13.110182.001125.
- ^Alboni, Paolo; Alboni, Marco; Bertorelle, Giorgio (2008). "The origin of vasovagal syncope: to protect the heart or to escape predation?".Clinical Autonomic Research.18(4): 170–8.doi:10.1007/s10286-008-0479-7.PMID18592129.S2CID7739227.
- ^abInman, Mason (29 March 2005)."Sea Hares Lose Their Lunch".Sciencemag.org.Archivedfrom the original on 2021-09-24.Retrieved10 May2015.
- ^Derby, Charles D. (December 2007). "Escape by Inking and Secreting: Marine Molluscs Avoid Predators Through a Rich Array of Chemicals and Mechanisms".The Biological Bulletin.213(3): 274–289.doi:10.2307/25066645.JSTOR25066645.PMID18083967.S2CID9539618.
- ^Derby, Charles D.; Kicklighter, Cynthia E.; Johnson, P. M. & Xu Zhang (29 March 2007)."Chemical Composition of Inks of Diverse Marine Molluscs Suggests Convergent Chemical Defenses"(PDF).Journal of Chemical Ecology.2007(33): 1105–1113.Bibcode:2007JCEco..33.1105D.doi:10.1007/s10886-007-9279-0.PMID17393278.S2CID92064.Archived fromthe original(PDF)on 15 November 2009.Retrieved9 May2015.
- ^Barrows, Edward M. (2001).Animal behavior(2nd ed.). CRC Press. p.177.ISBN978-0-8493-2005-7.
- ^abRuxton, Sherratt & Speed 2004,p. 198.
- ^Endler, J. A. (1981). "An overview of the relationships between mimicry and crypsis".Biological Journal of the Linnean Society.16:25–31.doi:10.1111/j.1095-8312.1981.tb01840.x.
- ^abcHolmgren, H.; Enquist, M. (1999)."Dynamics of mimicry evolution".Biological Journal of the Linnean Society.66(2): 145–158.Bibcode:1999BJLS...66..145H.doi:10.1006/bijl.1998.0269.
- ^abRitland, D. B. (1995). "Comparative unpalatability of mimetic viceroy butterflies (Limenitis archippus) from four south-eastern United States populations ".Oecologia.103(3): 327–336.Bibcode:1995Oecol.103..327R.doi:10.1007/BF00328621.PMID28306826.S2CID13436225.
- ^Bates, H. W.(1961)."Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae".Transactions of the Linnean Society.23(3): 495–566.doi:10.1111/j.1096-3642.1860.tb00146.x.
- ^Stearns, Stephen; Hoekstra, Rolf (2005).Evolution: An Introduction.Oxford University Press. p. 464.
- ^Norman, Mark; Finn, Julian; Tregenza, Tom (September 7, 2001)."Dynamic mimicry in an Indo-Malayan octopus".Proceedings: Biological Sciences.268(1478): 1755–1758.doi:10.1098/rspb.2001.1708.PMC1088805.PMID11522192.
- ^Hanlon, R.T.; Forsythe, J.W.; Joneschild, D.E. (1999)."Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on Indo-Pacific coral reefs, with a method of quantifying crypsis form video tapes".Biological Journal of the Linnean Society.66(1): 1–22.Bibcode:1999BJLS...66....1H.doi:10.1006/bijl.1998.0264.
- ^Holen, O.H.; Johnstone, R. A. (2004). "The Evolution of Mimicry under Constraints".The American Naturalist.164(5): 598–613.doi:10.1086/424972.PMID15540150.S2CID8153271.
- ^Norman, M.D.; Finn, J.; Tregenza, T. (2001)."Dynamic Mimicry in an Indo-Malayan Octopus".Proceedings: Biological Sciences.268(1478): 1755–1758.doi:10.1098/rspb.2001.1708.PMC1088805.PMID11522192.
- ^Müller, Fritz(1879). "ItunaandThyridia;a remarkable case of mimicry in butterflies. (R. Meldola translation.) ".Proclamations of the Entomological Society of London.1879:20–29.
- ^Thomas, Craig. Scott, Susan. (1997).All Stings Considered.University of Hawaii Press. pp. 96–97.
- ^abcVincent, J. F. V.; Owers, P. (1986). "Mechanical design of hedgehog spines and porcupine quills".Journal of Zoology.210:55–75.doi:10.1111/j.1469-7998.1986.tb03620.x.
- ^Warwick, Hugh (15 June 2014).Hedgehog.Reaktion Books. p. 10.ISBN978-1-78023-315-4.
- ^Murphy, Shannon M.; Leahy, Susannah M.; Williams, Laila S.; Lill, John T. (2010)."Stinging spines protect slug caterpillars (Limacodidae) from multiple generalist predators".Behavioral Ecology.21(1): 153–160.doi:10.1093/beheco/arp166.hdl:10.1093/beheco/arp166.
- ^Edmunds 1974,pp. 202–207.
- ^Duncan, P.; Vigne, N. (1979). "The effect of group size in horses on the rate of attacks by blood-sucking flies".Animal Behaviour.27:623–625.doi:10.1016/0003-3472(79)90201-x.S2CID53154054.
- ^Foster, W.A.; Treherne, J.E. (1981). "Evidence for the dilution effect in the selfish herd from fish predation on a marine insect".Nature.295(5832): 466–467.Bibcode:1981Natur.293..466F.doi:10.1038/293466a0.S2CID4365789.
- ^Hamilton, W.(1971). "Geometry for the selfish herd".Journal of Theoretical Biology.31(2): 295–311.Bibcode:1971JThBi..31..295H.doi:10.1016/0022-5193(71)90189-5.PMID5104951.
- ^De Vos, A.; O'Riain, M. J. (September 2009)."Sharks shape the geometry of a selfish seal herd: experimental evidence from seal decoys".Biology Letters.6(1): 48–50.doi:10.1098/rsbl.2009.0628.PMC2817263.PMID19793737.
- ^Williams, K.S. & C. Simon (1995)."The ecology, behavior, and evolution of periodical cicadas"(PDF).Annual Review of Entomology.40:269–295.doi:10.1146/annurev.en.40.010195.001413.
- ^Maynard Smith, John;Harper, David (2003).Animal Signals.Oxford University Press. pp. 113–121.ISBN978-0-19852-685-8.
- ^Caro 2005,pp. 115–149.
- ^Pulliam, H. R. (1973). "On the advantages of flocking".J. Theor. Biol.38(2): 419–22.Bibcode:1973JThBi..38..419P.doi:10.1016/0022-5193(73)90184-7.PMID4734745.
- ^Bertram, Brian C. (1980). "Vigilance and group size in ostriches".Animal Behaviour.28(1): 278–286.doi:10.1016/S0003-3472(80)80030-3.S2CID53144763.
- ^Stevens, M; Searle, WT; Seymour, JE; Marshall, KL; Ruxton, GD (2011)."Motion dazzle and camouflage as distinct anti-predator defenses".BMC Biol.9:81.doi:10.1186/1741-7007-9-81.PMC3257203.PMID22117898.
- ^abcRuxton, Sherratt & Speed 2004,pp. 64–69.
- ^abStanford, Craig B (1995). "The influence of chimpanzee predation on group size and anti-predator behavior in red colobus monkeys".Animal Behaviour.49(3): 577–587.doi:10.1016/0003-3472(95)90033-0.
- ^abcdWarham, John (1977)."The Incidence, Functions and Ecological Significance of Petrel Stomach Oils"(PDF).New Zealand Ecological Society.24:84–93.
- ^abcDavidson, D.W.; Salim, K.A.; Billen, J. (2011)."A review on self-destructive defense behaviors in social insects"(PDF).Insectes Sociaux.59:1–10.doi:10.1007/s00040-011-0210-x.S2CID13257903.
- ^abHulme, P. E.; Benkman, C. W. (2002). Herrera, C. M.; Pellmyr, O. (eds.).Granivory.Blackwell. pp. 132–154.ISBN978-0-632-05267-7.
{{cite book}}:|work=ignored (help) - ^abEdmunds 1974,pp. 189–201.
- ^Eisner, Thomas; Jones, Tappey H.; Aneshansley, Daniel J.; Tschinkel, Walter R.; Silberglied, Robert E.; Meinwald, Jerrold (1977). "Chemistry of defensive secretions of bombardier beetles (Brachinini, Metriini, Ozaenini, Paussini)".Journal of Insect Physiology.23(11–12): 1383–1386.doi:10.1016/0022-1910(77)90162-7.
- ^"See It to Believe It: Animals Vomit, Spurt Blood to Thwart Predators"Archived2017-09-14 at theWayback Machine,Allison Bond,Discover Magazineblog, 28 July 2009, retrieved 17 March 2010
- ^abcdHingston, R. W. G. (1927). "The liquid-squirting habit of oriental grasshoppers".Transactions of the Entomological Society of London.75:65–69.doi:10.1111/j.1365-2311.1927.tb00060.x.
- ^Young, B. A.; Dunlap, K.; Koenig, K.; Singer, M. (September 2004)."The buccal buckle: The functional morphology of venom spitting in cobras".Journal of Experimental Biology.207(20): 3483–3494.doi:10.1242/jeb.01170.PMID15339944.
- ^Mayell, Hillary (February 10, 2005)."Cobras Spit Venom at Eyes With Nearly Perfect Aim".National Geographic.Archived fromthe originalon November 10, 2005.
- ^O. Wilson, Edward(2000).Sociobiology: the New Synthesis.Harvard University Press.pp. 302–305.ISBN9780674000896.
- ^Sherbrooke, W.C. (2001). "Do vertebral-line patterns in two horned lizards (Phrynosoma spp.) mimic plant-stem shadows and stem litter?".J Arid Environ.50(1): 109–120.Bibcode:2002JArEn..50..109S.doi:10.1006/jare.2001.0852.
- ^Middendorf, George A.; Sherbrooke, Wade C. (1992)."Canid Elicitation of Blood-Squirting in a Horned Lizard (Phrynosoma Cornutum)".Copeia.1992(2): 519–27.doi:10.2307/1446212.JSTOR1446212.
- ^Pianka, Erika R. & Wendy L. Hodges."Horned Lizards".University of Texas. Archived fromthe originalon 29 April 2011.Retrieved18 November2013.
- ^abSherbrooke, Wade C.; George, A. Middendorf III; Douglas, M. E. (2004)."Responses of Kit Foxes (Vulpes macrotis) to Antipredator Blood-Squirting and Blood of Texas Horned Lizards (Phrynosoma cornutum) ".Copeia.2004(3): 652–658.doi:10.1643/ch-03-157r1.JSTOR1448486.S2CID55365586.
- ^Lim, Jeanette; Fudge, Douglas F.; Levy, Nimrod; Gosline, John M. (2006)."Hagfish Slime Ecomechanics: testing the gill-clogging mechanics".The Journal of Experimental Biology.209(Pt 4): 702–10.doi:10.1242/jeb.02067.PMID16449564.
- ^Andersson, Malte; Wiklund, Christer G. (1978). "Clumping versus spacing out: Experiments on nest predation in fieldfares (Turdus pilaris) ".Animal Behaviour.26(4): 1207–1212.doi:10.1016/0003-3472(78)90110-0.S2CID53195968.
- ^Parejo, D; Avilés, JM; Peña, A; Sánchez, L; Ruano, F; et al. (2013)."Armed Rollers: Does Nestling's Vomit Function as a Defence against Predators?".PLOS ONE.8(7): e68862.Bibcode:2013PLoSO...868862P.doi:10.1371/journal.pone.0068862.PMC3707886.PMID23874791.
- ^Peterson, Steven C., Nelson D. Johnson, and John L. LeGuyader (1987). "Defensive Regurgitation of Allelochemicals Derived From Host Cyanogenesis By Eastern Tent Caterpillars".Ecology.68(5): 1268–272.Bibcode:1987Ecol...68.1268P.doi:10.2307/1939211.JSTOR1939211.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Smedley, Scott R., Elizabeth Ehrhardt, and Thomas Eisner (1993)."Defensive Regurgitation by a Noctuid Moth Larva (Litoprosopus futilis) ".Psyche: A Journal of Entomology.100(3–4): 209–21.doi:10.1155/1993/67950.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Jones, T.H.; Clark, D.A.; Edwards, A.; Davidson, D.W.; Spande, T.F.; Snelling, R.R. (2004). "The chemistry of exploding ants,Camponotusspp. (Cylindricus complex) ".Journal of Chemical Ecology.30(8): 1479–1492.Bibcode:2004JCEco..30.1479J.doi:10.1023/b:joec.0000042063.01424.28.PMID15537154.S2CID23756265.
- ^abCorlett, Richard T.; Primack, Richard B. (2011).Tropical rain forests: an ecological and biogeographical comparison(2nd ed.). Wiley-Blackwell. pp. 197, 200.ISBN978-1444332551.
- ^Bromley, Richard G. (2012).Trace Fossils: Biology, Taxonomy and Applications.Routledge. pp. 69–72.ISBN978-1-135-07607-8.
- ^Kruszelnicki, Karl S. (August 9, 1999)."Real Wheel Animals—Part Two".Great Moments in Science.ABC Science.Archivedfrom the original on October 1, 2016.
- ^abcdEdmunds 1974,pp. 145–149.
- ^Edmunds 1974,pp. 179–181.
- ^Aristotle(1910) [350 BC].The History of Animals.Vol. IX. pp. 621b–622a.
- ^Geffroy, Benjamin; Sadoul, Bastien; Putman, Breanna J.; Berger-Tal, Oded; Garamszegi, László Zsolt; Møller, Anders Pape; Blumstein, Daniel T. (22 September 2020)."Evolutionary dynamics in the Anthropocene: Life history and intensity of human contact shape antipredator responses".PLOS Biology.18(9): e3000818.doi:10.1371/journal.pbio.3000818.ISSN1545-7885.PMC7508406.PMID32960897.S2CID221864354.
Sources[edit]
- Caro, Tim(2005).Antipredator Defenses in Birds and Mammals.University of Chicago Press.
- Cott, Hugh(1940).Adaptive Coloration in Animals.Oxford University Press.
- Edmunds, Malcolm (1974).Defence in Animals.Longman.
- Ruxton, Graeme D.;Sherratt, Thomas N.;Speed, Michael P. (2004).Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry.Oxford.
