Biological network
| Part ofa serieson | ||||
| Network science | ||||
|---|---|---|---|---|
| Network types | ||||
| Graphs | ||||
|
||||
| Models | ||||
|
||||
| ||||
This articleneeds additional citations forverification.(October 2011) |
Abiological networkis a method of representing systems as complex sets of binary interactions or relations between various biological entities.[1]In general, networks or graphs are used to capture relationships between entities or objects.[1]A typicalgraphingrepresentation consists of a set ofnodesconnected byedges.

History of networks
[edit]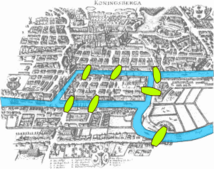
As early as 1736Leonhard Euleranalyzed a real-world issue known as theSeven Bridges of Königsberg,which established the foundation ofgraph theory.From the 1930s-1950s the study ofrandom graphswere developed. During the mid 1990s, it was discovered that many different types of "real" networks have structural properties quite different from random networks.[2]In the late 2000's, scale-free and small-world networks began shaping the emergence of systems biology, network biology, and network medicine.[1]In 2014, graph theoretical methods were used byFrank Emmert-Streibto analyze biological networks.
In the 1980s, researchers started viewingDNAorgenomesas the dynamic storage of a language system with precise computable finitestatesrepresented as afinite state machine.[3]Recentcomplex systemsresearch has also suggested some far-reaching commonality in the organization of information in problems from biology,computer science,andphysics.
Networks in biology
[edit]Protein–protein interaction networks
[edit]
Protein-protein interaction networks(PINs) represent the physical relationship among proteins present in a cell, where proteins arenodes,and their interactions are undirectededges.[4]Due to their undirected nature, it is difficult to identify all the proteins involved in an interaction.Protein–protein interactions(PPIs) are essential to the cellular processes and also the most intensely analyzed networks in biology. PPIs could be discovered by various experimental techniques, among which theyeast two-hybrid systemis a commonly used technique for the study of binary interactions.[5]Recently, high-throughput studies using mass spectrometry have identified large sets of protein interactions.[6]
Many international efforts have resulted in databases that catalog experimentally determined protein-protein interactions. Some of them are theHuman Protein Reference Database,Database of Interacting Proteins,the Molecular Interaction Database (MINT),[7]IntAct,[8]andBioGRID.[9]At the same time, multiple computational approaches have been proposed to predict interactions.[10]FunCoupandSTRINGare examples of such databases, where protein-protein interactions inferred from multiple evidences are gathered and made available for public usage.
Recent studies have indicated the conservation of molecular networks through deep evolutionary time.[11]Moreover, it has been discovered that proteins with high degrees of connectedness are more likely to be essential for survival than proteins with lesser degrees.[12]This observation suggests that the overall composition of the network (not simply interactions between protein pairs) is vital for an organism's overall functioning.
Gene regulatory networks (DNA–protein interaction networks)
[edit]
Thegenomeencodes thousands of genes whose products (mRNAs,proteins) are crucial to the various processes of life, such as cell differentiation, cell survival, and metabolism. Genes produce such products through a process called transcription, which is regulated by a class of proteins calledtranscription factors.For instance, the human genome encodes almost 1,500 DNA-binding transcription factors that regulate the expression of more than 20,000 human genes.[13]The complete set of gene products and the interactions among them constitutesgene regulatory networks(GRN). GRNs regulate the levels of gene products within the cell and in-turn the cellular processes.
GRNs are represented with genes and transcriptional factors as nodes and the relationship between them as edges. These edges are directional, representing the regulatory relationship between the two ends of the edge. For example., the directed edge from gene A to gene B indicates that A regulates the expression of B. Thus, these directional edges can not only represent the promotion of gene regulation but also its inhibition.
GRNs are usually constructed by utilizing the gene regulation knowledge available from databases such as.,ReactomeandKEGG.High-throughput measurement technologies, such asmicroarray,RNA-Seq,ChIP-chip,andChIP-seq,enabled the accumulation of large-scale transcriptomics data, which could help in understanding the complex gene regulation patterns.[14][15]
Gene co-expression networks (transcript–transcript association networks)
[edit]Gene co-expression networks can be perceived as association networks between variables that measure transcript abundances. These networks have been used to provide a system biologic analysis of DNA microarray data, RNA-seq data, miRNA data, etc.weighted gene co-expression network analysisis extensively used to identify co-expression modules and intramodular hub genes.[16]Co-expression modules may correspond to cell types or pathways, while highly connected intramodular hubs can be interpreted as representatives of their respective modules.
Metabolic networks
[edit]
Cells break down the food and nutrients into small molecules necessary for cellular processing through a series of biochemical reactions. These biochemical reactions are catalyzed byenzymes.The complete set of all these biochemical reactions in all the pathways represents themetabolic network.Within the metabolic network, the small molecules take the roles of nodes, and they could be either carbohydrates, lipids, or amino acids. The reactions which convert these small molecules from one form to another are represented as edges. It is possible to use network analyses to infer how selection acts on metabolic pathways.[17]
Signaling networks
[edit]
Signals are transduced within cells or in between cells and thus form complex signaling networks which plays a key role in the tissue structure. For instance, theMAPK/ERK pathwayis transduced from the cell surface to the cell nucleus by a series of protein-protein interactions, phosphorylation reactions, and other events.[18]Signaling networks typically integrateprotein–protein interaction networks,gene regulatory networks,andmetabolic networks.[19][20]Single cell sequencing technologies allows the extraction of inter-cellular signaling, an example is NicheNet, which allows to modeling intercellular communication by linking ligands to target genes.[21]
Neuronal networks
[edit]The complex interactions in thebrainmake it a perfect candidate to apply network theory.Neuronsin the brain are deeply connected with one another, and this results in complex networks being present in the structural and functional aspects of the brain.[22]For instance,small-world networkproperties have been demonstrated in connections between cortical regions of the primate brain[23]or during swallowing in humans.[24]This suggests that cortical areas of the brain are not directly interacting with each other, but most areas can be reached from all others through only a few interactions.
Food webs
[edit]All organisms are connected through feeding interactions. If a species eats or is eaten by another species, they are connected in an intricatefood webof predator and prey interactions. The stability of these interactions has been a long-standing question in ecology.[25]That is to say if certain individuals are removed, what happens to the network (i.e., does it collapse or adapt)? Network analysis can be used to explore food web stability and determine if certain network properties result in more stable networks. Moreover, network analysis can be used to determine how selective removals of species will influence the food web as a whole.[26]This is especially important considering the potential species loss due to global climate change.
Between-species interaction networks
[edit]In biology, pairwise interactions have historically been the focus of intense study. With the recent advances innetwork science,it has become possible to scale up pairwise interactions to include individuals of many species involved in many sets of interactions to understand the structure and function of largerecological networks.[27]The use ofnetwork analysiscan allow for both the discovery and understanding of how these complex interactions link together within the system's network, a property that has previously been overlooked. This powerful tool allows for the study of various types of interactions (fromcompetitivetocooperative) using the same general framework.[28]For example, plant-pollinatorinteractions are mutually beneficial and often involve many different species of pollinators as well as many different species of plants. These interactions are critical to plant reproduction and thus the accumulation of resources at the base of thefood chainfor primary consumers, yet these interaction networks are threatened byanthropogenicchange. The use of network analysis can illuminate howpollination networkswork and may, in turn, inform conservation efforts.[29]Within pollination networks, nestedness (i.e., specialists interact with a subset of species that generalists interact with), redundancy (i.e., most plants are pollinated by many pollinators), andmodularityplay a large role in network stability.[29][30]These network properties may actually work to slow the spread of disturbance effects through the system and potentially buffer the pollination network from anthropogenic changes somewhat.[30]More generally, the structure of species interactions within an ecological network can tell us something about the diversity, richness, and robustness of the network.[31]Researchers can even compare current constructions of species interactions networks with historical reconstructions of ancient networks to determine how networks have changed over time.[32]Much research into these complex species interactions networks is highly concerned with understanding what factors (e.g., species richness, connectance, nature of the physical environment) lead to network stability.[33][34]
Within-species interaction networks
[edit]Network analysis provides the ability to quantify associations between individuals, which makes it possible to infer details about the network as a whole at the species and/or population level.[35]One of the most attractive features of the network paradigm would be that it provides a single conceptual framework in which the social organization of animals at all levels (individual, dyad, group, population) and for all types of interaction (aggressive, cooperative, sexual, etc.) can be studied.[36]
Researchers interested inethologyacross many taxa, from insects to primates, are starting to incorporate network analysis into their research. Researchers interested in social insects (e.g., ants and bees) have used network analyses better to understand the division of labor, task allocation, and foraging optimization within colonies.[37][38][39]Other researchers are interested in how specific network properties at the group and/or population level can explain individual-level behaviors. Studies have demonstrated how animal social network structure can be influenced by factors ranging from characteristics of the environment to characteristics of the individual, such as developmental experience and personality. At the level of the individual, the patterning of social connections can be an important determinant offitness,predicting both survival and reproductive success. At the population level, network structure can influence the patterning of ecological and evolutionary processes, such asfrequency-dependent selectionand disease and information transmission.[40]For instance, a study onwire-tailed manakins(a small passerine bird) found that a male'sdegreein the network largely predicted the ability of the male to rise in the social hierarchy (i.e., eventually obtain a territory and matings).[41]Inbottlenose dolphingroups, an individual's degree andbetweenness centralityvalues may predict whether or not that individual will exhibit certain behaviors, like the use of side flopping and upside-down lobtailing to lead group traveling efforts; individuals with high betweenness values are more connected and can obtain more information, and thus are better suited to lead group travel and therefore tend to exhibit these signaling behaviors more than other group members.[42]
Social network analysiscan also be used to describe the social organization within a species more generally, which frequently reveals important proximate mechanisms promoting the use of certain behavioral strategies. These descriptions are frequently linked to ecological properties (e.g., resource distribution). For example, network analyses revealed subtle differences in the group dynamics of two related equidfission-fusionspecies,Grevy's zebraandonagers,living in variable environments; Grevy's zebras show distinct preferences in their association choices when they fission into smaller groups, whereas onagers do not.[43]Similarly, researchers interested in primates have also utilized network analyses to compare social organizations across the diverseprimateorder, suggesting that using network measures (such ascentrality,assortativity,modularity,and betweenness) may be useful in terms of explaining the types of social behaviors we see within certain groups and not others.[44]
Finally, social network analysis can also reveal important fluctuations in animal behaviors across changing environments. For example, network analyses in femalechacma baboons(Papio hamadryas ursinus) revealed important dynamic changes across seasons that were previously unknown; instead of creating stable, long-lasting social bonds with friends, baboons were found to exhibit more variable relationships which were dependent on short-term contingencies related to group-level dynamics as well as environmental variability.[45]Changes in an individual's social network environment can also influence characteristics such as 'personality': for example, social spiders that huddle with bolder neighbors tend to increase also in boldness.[46]This is a very small set of broad examples of how researchers can use network analysis to study animal behavior. Research in this area is currently expanding very rapidly, especially since the broader development of animal-borne tags andcomputer visioncan be used to automate the collection of social associations.[47]Social network analysis is a valuable tool for studying animal behavior across all animal species and has the potential to uncover new information about animal behavior and social ecology that was previously poorly understood.
DNA-DNA chromatin networks
[edit]Within a nucleus,DNAis constantly in motion. Perpetual actions such as genome folding and Cohesin extrusion morph the shape of a genome in real time. The spatial location of strands ofchromatinrelative to each other plays an important role in the activation or suppression of certain genes. DNA-DNA Chromatin Networks help biologists to understand these interactions by analyzing commonalities amongst differentloci.The size of a network can vary significantly, from a few genes to several thousand and thus network analysis can provide vital support in understanding relationships among different areas of the genome. As an example, analysis of spatially similar loci within the organization in a nucleus withGenome Architecture Mapping (GAM)can be used to construct a network of loci with edges representing highly linked genomic regions.
The first graphic showcases the Hist1 region of the mm9 mouse genome with each node representing genomic loci. Two nodes are connected by an edge if their linkage disequilibrium is greater than the average across all 81 genomic windows. The locations of the nodes within the graphic are randomly selected and the methodology of choosing edges yields a, simple to show, but rudimentary graphical representation of the relationships in the dataset. The second visual exemplifies the same information as the previous; However, the network starts with every loci placed sequentially in a ring configuration. It then pulls nodes together using linear interpolation by their linkage as a percentage. The figure illustrates strong connections between the center genomic windows as well as the edge loci at the beginning and end of the Hist1 region.
Modelling biological networks
[edit]Introduction
[edit]To draw useful information from a biological network, an understanding of the statistical and mathematical techniques of identifying relationships within a network is vital. Procedures to identify association, communities, and centrality within nodes in a biological network can provide insight into the relationships of whatever the nodes represent whether they are genes, species, etc. Formulation of these methods transcends disciplines and relies heavily ongraph theory,computer science,andbioinformatics.
Association
[edit]
There are many different ways to measure the relationships of nodes when analyzing a network. In many cases, the measure used to find nodes that share similarity within a network is specific to the application it is being used. One of the types of measures that biologists utilize iscorrelationwhich specifically centers around the linear relationship between two variables.[48]As an example,weighted gene co-expression network analysisusesPearson correlationto analyze linked gene expression and understand genetics at a systems level.[49]Another measure of correlation islinkage disequilibrium.Linkage disequilibrium describes the non-random association of genetic sequences among loci in a given chromosome.[50]An example of its use is in detecting relationships inGAMdata across genomic intervals based upon detection frequencies of certain loci.[51]
Centrality
[edit]The concept ofcentralitycan be extremely useful when analyzing biological network structures. There are many different methods to measure centrality such as betweenness, degree, Eigenvector, and Katz centrality. Every type of centrality technique can provide different insights on nodes in a particular network; However, they all share the commonality that they are to measure the prominence of a node in a network.[52] In 2005, Researchers atHarvard Medical Schoolutilized centrality measures with the yeast protein interaction network. They found that proteins that exhibited high Betweenness centrality were more essential and translated closely to a given protein's evolutionary age.[53]
Communities
[edit]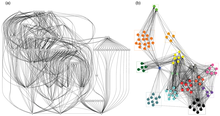
Studying thecommunity structureof a network by subdividing groups of nodes into like-regions can be an integral tool for bioinformatics when exploring data as a network.[54]A food web of TheSecaucus High SchoolMarsh exemplifies the benefits of grouping as the relationships between nodes are far easier to analyze with well-made communities. While the first graphic is hard to visualize, the second provides a better view of the pockets of highly connected feeding relationships that would be expected in a food web. The problem of community detection is still an active problem. Scientists and graph theorists continuously discover new ways of sub sectioning networks and thus a plethora of differentalgorithmsexist for creating these relationships.[55]Like many other tools that biologists utilize to understand data with network models, every algorithm can provide its own unique insight and may vary widely on aspects such as accuracy ortime complexityof calculation. In 2002, a food web of marine mammals in theChesapeake Baywas divided into communities by biologists using a community detection algorithm based on neighbors of nodes with high degree centrality. The resulting communities displayed a sizable split in pelagic and benthic organisms.[56]Two very common community detection algorithms for biological networks are the Louvain Method and Leiden Algorithm.
TheLouvain methodis agreedy algorithmthat attempts to maximizemodularity,which favors heavy edges within communities and sparse edges between, within a set of nodes. The algorithm starts by each node being in its own community and iteratively being added to the particular node's community that favors a higher modularity.[57][58]Once no modularity increase can occur by joining nodes to a community, a newweighted networkis constructed of communities as nodes with edges representing between-community edges and loops representing edges within a community. The process continues until no increase in modularity occurs.[59]While the Louvain Method provides good community detection, there are a few ways that it is limited. By mainly focusing on maximizing a given measure of modularity, it may be led to craft badly connected communities by degrading a model for the sake of maximizing a modularity metric; However, the Louvain Method performs fairly and is can be easy to understand comparatively to many other community detection algorithms.[58]
The Leiden Algorithm expands on the Louvain Method by providing a number of improvements. When joining nodes to a community, only neighborhoods that have been recently changed are considered. This greatly improves the speed of merging nodes. Another optimization is in the refinement phase in-which the algorithm randomly chooses for a node from a set of communities to merge with. This allows for greater depth in choosing communities as Louvain solely focuses on maximizing the modularity that was chosen. The Leiden algorithm, while more complex than Louvain, performs faster with better community detection and can be a valuable tool for identifying groups.[58]
Network Motifs
[edit]Network motifs,or statistically significant recurring interaction patterns within a network, are a commonly used tool to understand biological networks. A major use case of network motifs is inNeurophysiologywhere motif analysis is commonly used to understand interconnected neuronal functions at varying scales.[60]As an example, in 2017, researchers atBeijing Normal Universityanalyzed highly represented 2 and 3 node network motifs in directed functional brain networks constructed byResting state fMRIdata to study the basic mechanisms in brain information flow.[61]
See also
[edit]- List of omics topics in biology
- Biological network inference
- Biostatistics
- Computational biology
- Systems biology
- Weighted correlation network analysis
- Interactome
- Network medicine
- Ecological network
References
[edit]- ^abKoutrouli, Mikaela; Karatzas, Evangelos; Paez-Espino, David; Pavlopoulos, Georgios A. (2020)."A Guide to Conquer the Biological Network Era Using Graph Theory".Frontiers in Bioengineering and Biotechnology.8:34.doi:10.3389/fbioe.2020.00034.PMC7004966.PMID32083072.
- ^Emmert-Streib, Frank; Dehmer, Matthias (2015)."Biological networks: the microscope of the twenty-first century?".Frontiers in Genetics.6:307.doi:10.3389/fgene.2015.00307.PMC4602153.PMID26528327.
- ^Searls, D.B. (1993). "The computational linguistics of biological sequences".Artificial intelligence and molecular biology.Cambridge, MA: MIT Press.ISBN978-0-262-58115-8.OCLC77932373.
- ^Habibi, Iman; Emamian, Effat S.; Abdi, Ali (2014-01-01)."Quantitative analysis of intracellular communication and signaling errors in signaling networks".BMC Systems Biology.8:89.doi:10.1186/s12918-014-0089-z.PMC4255782.PMID25115405.
- ^Mashaghi, A.; et al. (2004). "Investigation of a protein complex network".European Physical Journal.41(1): 113–121.arXiv:cond-mat/0304207.Bibcode:2004EPJB...41..113M.doi:10.1140/epjb/e2004-00301-0.S2CID9233932.
- ^Smits, Arne H.; Vermeulen, Michiel (2016). "Characterizing Protein–Protein Interactions Using Mass Spectrometry: Challenges and Opportunities".Trends in Biotechnology.34(10): 825–834.doi:10.1016/j.tibtech.2016.02.014.hdl:2066/161800.ISSN0167-7799.PMID26996615.
- ^Zanzoni, A; Montecchi-Palazzi, L; Quondam, M; Ausiello, G; Helmer-Citterich, M; Cesareni, G (Feb 20, 2002)."MINT: a Molecular INTeraction database".FEBS Letters.513(1): 135–40.doi:10.1016/s0014-5793(01)03293-8.PMC1751541.PMID11911893.
- ^Kerrien, S.; Aranda, B.; Breuza, L.; Bridge, A.; Broackes-Carter, F.; Chen, C.; Duesbury, M.; Dumousseau, M.; Feuermann, M.; Hinz, U.; Jandrasits, C.; Jimenez, R. C.; Khadake, J.; Mahadevan, U.; Masson, P.; Pedruzzi, I.; Pfeiffenberger, E.; Porras, P.; Raghunath, A.; Roechert, B.; Orchard, S.; Hermjakob, H. (24 November 2011)."The IntAct molecular interaction database in 2012".Nucleic Acids Research.40(D1): D841–D846.doi:10.1093/nar/gkr1088.PMC3245075.PMID22121220.
- ^Oughtred, Rose; Rust, Jennifer; Chang, Christie; Breitkreutz, Bobby-Joe; Stark, Chris; Willems, Andrew; Boucher, Lorrie; Leung, Genie; Kolas, Nadine; Zhang, Frederick; Dolma, Sonam; Coulombe-Huntington, Jasmin; Chatr-aryamontri, Andrew; Dolinski, Kara; Tyers, Mike (2020)."TheBioGRIDdatabase: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions".Protein Science.30(1): 187–200.doi:10.1002/pro.3978.PMC7737760.PMID33070389.
- ^Jansen, R. (2003). "A Bayesian Networks Approach for Predicting Protein-Protein Interactions from Genomic Data".Science.302(5644): 449–453.Bibcode:2003Sci...302..449J.doi:10.1126/science.1087361.ISSN0036-8075.PMID14564010.S2CID5293611.
- ^Sharan, R.; et al. (2005)."Conserved patterns of protein interaction in multiple species".Proceedings of the National Academy of Sciences of the United States of America.102(6): 1974–9.Bibcode:2005PNAS..102.1974S.doi:10.1073/pnas.0409522102.PMC548573.PMID15687504.
- ^Jeong, H.; et al. (2001). "Lethality and centrality in protein networks".Nature.411(6833): 41–42.arXiv:cond-mat/0105306.Bibcode:2001Natur.411...41J.doi:10.1038/35075138.PMID11333967.S2CID258942.
- ^Vaquerizas, J.-M.; et al. (2009). "A census of human transcription factors: function, expression and evolution".Nature Reviews Genetics.10(4): 252–263.doi:10.1038/nrg2538.PMID19274049.S2CID3207586.
- ^Jia, Bochao; Xu, Suwa; Xiao, Guanghua; Lamba, Vishal; Liang, Faming (2017)."Learning gene regulatory networks from next generation sequencing data".Biometrics.73(4): 1221–30.doi:10.1111/biom.12682.PMC6258556.PMID28294287.
- ^Angelini, Claudia; Costa, Valerio (2014)."Understanding gene regulatory mechanisms by integrating ChIP-seq and RNA-seq data: statistical solutions to biological problems".Frontiers in Cell and Developmental Biology.2:51.doi:10.3389/fcell.2014.00051.PMC4207007.PMID25364758.
- ^Zheng, Peng-Fei; Chen, Lu-Zhu; Guan, Yao-Zong; Liu, Peng (2021)."Weighted gene co-expression network analysis identifies specific modules and hub genes related to coronary artery disease".Scientific Reports.11(1): 6711.Bibcode:2021NatSR..11.6711Z.doi:10.1038/s41598-021-86207-0.PMC7988178.PMID33758323.
- ^Proulx, S.R.; et al. (2005). "Network thinking in ecology and evolution".Trends in Ecology and Evolution.20(6): 345–353.doi:10.1016/j.tree.2005.04.004.PMID16701391.
- ^Cargnello, M.; Roux, P. P. (2011)."Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases".Microbiology and Molecular Biology Reviews.75(1): 50–83.doi:10.1128/MMBR.00031-10.PMC3063353.PMID21372320.
- ^Sevimoglu, Tuba; Arga, Kazim Yalcin (2014)."The role of protein interaction networks in systems biomedicine".Computational and Structural Biotechnology Journal.11(18): 22–27.doi:10.1016/j.csbj.2014.08.008.PMC4212283.PMID25379140.
- ^Arga, K Yalçın; Önsan, Z İlsen; Kırdar, Betül; Ülgen, Kutlu Ö; Nielsen, Jens (2007). "Understanding signaling in yeast: Insights from network analysis".Biotechnology and Bioengineering.97(5): 1246–58.doi:10.1002/bit.21317.ISSN0006-3592.PMID17252576.S2CID38896124.
- ^Browaeys, Robin; Saelens, Wouter; Saeys, Yvan (February 2020). "NicheNet: modeling intercellular communication by linking ligands to target genes".Nature Methods.17(2): 159–162.doi:10.1038/s41592-019-0667-5.PMID31819264.S2CID256836571.
- ^Bullmore, E. & O. Sporns (2009). "Complex brain networks: graph theoretical analysis of structural and functional systems".Nature Reviews Neuroscience.10(3): 186–198.doi:10.1038/nrn2575.PMID19190637.S2CID205504722.
- ^Stephan, K.E.; et al. (2000)."Computational analysis of functional connectivity between areas of primate cerebral cortex".Philosophical Transactions of the Royal Society B.355(1393): 111–126.doi:10.1098/rstb.2000.0552.PMC1692715.PMID10703047.
- ^Jestrović, Iva; Coyle, James L; Perera, Subashan; Sejdić, Ervin (2016-12-01)."Functional connectivity patterns of normal human swallowing: difference among various viscosity swallows in normal and chin-tuck head positions".Brain Research.1652:158–169.doi:10.1016/j.brainres.2016.09.041.ISSN0006-8993.PMC5102805.PMID27693396.
- ^MacArthur, R.H.(1955). "Fluctuations in animal populations and a measure of community stability".Ecology.36(3): 533–6.Bibcode:1955Ecol...36..533M.doi:10.2307/1929601.JSTOR1929601.
- ^Dunne, J.A.; et al. (2002). "Network structure and biodiversity loss in food webs: robustness increases with connectance".Ecology Letters.5(4): 558–567.Bibcode:2002EcolL...5..558D.doi:10.1046/j.1461-0248.2002.00354.x.S2CID2114852.
- ^Bascompte, J. (2009). "Disentangling the web of life".Science.325(5939): 416–9.Bibcode:2009Sci...325..416B.doi:10.1126/science.1170749.PMID19628856.S2CID2249052.
- ^Krause, J.; et al. (2009). "Animal social networks: an introduction".Behav. Ecol. Sociobiol.63(7): 967–973.doi:10.1007/s00265-009-0747-0.S2CID24523607.
- ^abMemmott, J.; et al. (2004)."Tolerance of pollination networks to species extinctions".Philosophical Transactions of the Royal Society B.271(1557): 2605–261.doi:10.1098/rspb.2004.2909.PMC1691904.PMID15615687.
- ^abOlesen, J.; et al. (2007)."The modularity of pollination networks".PNAS.104(50): 19891–6.Bibcode:2007PNAS..10419891O.doi:10.1073/pnas.0706375104.PMC2148393.PMID18056808.
- ^Campbell, V.; et al. (2011). "Experimental design and the outcome and interpretation of diversity-stability relations".Oikos.120(3): 399–408.Bibcode:2011Oikos.120..399C.doi:10.1111/j.1600-0706.2010.18768.x.
- ^Lotze, H.; et al. (2011). "Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean".Ecosystems.14(2): 198–222.Bibcode:2011Ecosy..14..198L.doi:10.1007/s10021-010-9404-8.S2CID45894582.
- ^Romanuk, T.; et al. (2010)."Maintenance of positive diversity-stability relations along a gradient of environmental stress".PLOS ONE.5(4): e10378.Bibcode:2010PLoSO...510378R.doi:10.1371/journal.pone.0010378.PMC2860506.PMID20436913.
- ^Briand, F. (1983). "Environmental control of food web structure".Ecology.64(2): 253–263.Bibcode:1983Ecol...64..253B.doi:10.2307/1937073.JSTOR1937073.
- ^Croft, D.P.; et al. (2004)."Social networks in the guppy (Poecilia reticulate)".Philosophical Transactions of the Royal Society B.271(Suppl): S516–S519.doi:10.1098/rsbl.2004.0206.PMC1810091.PMID15801620.
- ^Krause, Jens; Lusseau, David; James, Richard (1 May 2009)."Animal social networks: an introduction".Behavioral Ecology and Sociobiology.63(7): 967–973.doi:10.1007/s00265-009-0747-0.S2CID24523607.
- ^Dornhaus, A.; et al. (2006)."Benefits of recruitment in honey bees: Effects of ecology and colony size in an individual-based model".Behavioral Ecology.17(3): 336–344.doi:10.1093/beheco/arj036.
- ^Linksvayer, T.; et al. (2012). "Developmental evolution in social insects: Regulatory networks from genes to societies".Journal of Experimental Zoology Part B: Molecular and Developmental Evolution.318(3): 159–169.Bibcode:2012JEZB..318..159L.doi:10.1002/jez.b.22001.PMID22544713.
- ^Mullen, R.; et al. (2009). "A review of ant algorithms".Expert Systems with Applications.36(6): 9608–17.doi:10.1016/j.eswa.2009.01.020.
- ^Croft, Darren P.; Darden, Safi K.; Wey, Tina W. (2016)."Current directions in animal social networks".Current Opinion in Behavioral Sciences.12:52–58.doi:10.1016/j.cobeha.2016.09.001.hdl:10871/23348.S2CID53195734.
- ^Ryder, T.B.; et al. (2008)."Social networks in the lek-mating wire-tailed manakin (Pipra filicauda) ".Philosophical Transactions of the Royal Society B.275(1641): 1367–74.doi:10.1098/rspb.2008.0205.PMC2602714.PMID18381257.
- ^Lusseau, D. (2007). "Evidence for social role in a dolphin social network".Evolutionary Ecology.21(3): 357–366.arXiv:q-bio/0607048.Bibcode:2007EvEco..21..357L.doi:10.1007/s10682-006-9105-0.S2CID9748737.
- ^Sundaresan, S.; et al. (2007). "Network metrics reveal differences in social organization between two fission-fusion species, Grevy's zebra and onager".Oecologia.151(1): 140–9.Bibcode:2007Oecol.151..140S.doi:10.1007/s00442-006-0553-6.PMID16964497.S2CID8104281.
- ^Kasper, C.; Voelkl, B. (2009). "A social network analysis of primate groups".Primates.50(4): 343–356.doi:10.1007/s10329-009-0153-2.PMID19533270.S2CID9852394.
- ^Henzi, S.; et al. (2009). "Cyclicity in the structure of female baboon social networks".Behavioral Ecology and Sociobiology.63(7): 1015–21.doi:10.1007/s00265-009-0720-y.S2CID6021233.
- ^Hunt, E.R.; et al. (2018)."Social interactions shape individual and collective personality in social spiders".Proceedings of the Royal Society B.285(1886): 20181366.doi:10.1098/rspb.2018.1366.PMC6158534.PMID30185649.
- ^Krause J, Krause S, Arlinghaus R, Psorakis I, Roberts S, Rutz C (2013)."Reality mining of animal social systems".Trends in Ecology and Evolution.28(9): 541–551.doi:10.1016/j.tree.2013.06.002.PMID23856617.Archivedfrom the original on 2023-02-04.Retrieved2019-12-08.
- ^Haug, Mark Gerard.measure of association.Encyclopedia Britannica.
- ^Zhang, Bin; Horvath, Steve (2005)."A General Framework for Weighted Gene Co-Expression Network Analysis"(PDF).Statistical Applications in Genetics and Molecular Biology.4(1): 17.doi:10.2202/1544-6115.1128.PMID16646834.
- ^"Linkage Disequilibrium".ISOGG Wiki.International Society of Genetic Genealogy.
- ^Beagrie RA, Scialdone A, Schueler M, Kraemer DC, Chotalia M, Xie SQ, Barbieri M, de Santiago I, Lavitas LM, Branco MR, Fraser J, Dostie J, Game L, Dillon N, Edwards PA, Nicodemi M, Pombo A (March 2017)."Complex multi-enhancer contacts captured by genome architecture mapping".Nature.543(7646): 519–524.doi:10.1038/nature21411.PMC5366070.PMID28273065.
- ^"Centrality Measure — an Overview".ScienceDirect Topics.ScienceDirect.
- ^Joy MP, Brock A, Ingber DE, Huang S (June 2005)."High-betweenness proteins in the yeast protein interaction network".J Biomed Biotechnol.2005(2): 96–103.doi:10.1155/JBB.2005.96.PMC1184047.PMID16046814.
- ^Porter, Mason A.; et al. (2009)."Communities in Networks"(PDF).Notices of the AMS.56(9): 1082–97.
- ^"Community Detection — an Overview".ScienceDirect Topics.ScienceDirect.
- ^Girvan M, Newman ME (June 2002)."Community structure in social and biological networks".Proc Natl Acad Sci U S A.99(12): 7821–6.doi:10.1073/pnas.122653799.PMC122977.PMID12060727.
- ^Markovitch O, Krasnogor N (2018)."Predicting species emergence in simulated complex pre-biotic networks".PLOS ONE.13(2): e0192871.doi:10.1371/journal.pone.0192871.PMC5813963.PMID29447212.
- ^abcTraag VA, Waltman L, van Eck NJ (March 2019)."From Louvain to Leiden: guaranteeing well-connected communities".Sci Rep.9(1): 5233.doi:10.1038/s41598-019-41695-z.PMC6435756.PMID30914743.
- ^Ozaki, Naoto; Hiroshi, Tezuka; Inaba, Mary (3 June 2016)."A Simple Acceleration Method for the Louvain Algorithm"(PDF).International Journal of Computer and Electrical Engineering.8(3): 207–218.doi:10.17706/IJCEE.2016.8.3.207-218.
- ^Mittal D, Narayanan R (July 2024)."Network motifs in cellular neurophysiology".Trends Neurosci.47(7): 506–521.doi:10.1016/j.tins.2024.04.008.PMID38806296.
- ^Wei Y, Liao X, Yan C, He Y, Xia M (May 2017)."Identifying topological motif patterns of human brain functional networks".Hum Brain Mapp.38(5): 2734–50.doi:10.1002/hbm.23557.PMC6866742.PMID28256774.
Books
[edit]- Estrada, E. (2011).The Structure of Complex Networks: Theory and Applications.Oxford University Press.doi:10.1093/acprof:oso/9780199591756.001.0001.ISBN978-0-199-59175-6.OCLC780445324.
- Krause, J.; James, R.; Franks, D.; Croft, D. (2015).Animal Social Networks.Oxford University Press.doi:10.1093/acprof:oso/9780199679041.001.0001.ISBN978-0-19-967904-1.OCLC900722740.
External links
[edit]- Networkbio.org,The site of the series of Integrative Network Biology (INB) meetings. For the 2012 event also see networkbio.org
- Network Tools and Applications in Biology(NETTAB) workshops.
- Networkbiology.org,NetworkBiology wiki site.
- Linding Lab,Technical University of Denmark (DTU) studies Network Biology and Cellular Information Processing, and is also organizing the Denmark branch of the annual "Integrative Network Biology and Cancer"symposium series.
- NRNB.org,The National Resource for Network Biology. A US National Institute of Health (NIH) Biomedical Technology Research Center dedicated to the study of biological networks.
- Network RepositoryThe first interactive data and network data repository with real-time visual analytics.
- Animal Social Network Repository (ASNR)The first multi-taxonomic repository that collates 790 social networks from more than 45 species, including those of mammals, reptiles, fish, birds, and insects