Biosynthesis
This article has multiple issues.Please helpimprove itor discuss these issues on thetalk page.(Learn how and when to remove these messages)
|
Biosynthesis,i.e.,chemical synthesisoccurring in biological contexts, is a term most often referring to multi-step,enzyme-catalyzedprocesses where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzymesubstrates,with conversion by the living organism either into simpler or more complexproducts.Examples of biosynthetic pathways include those for the production ofamino acids,lipid membranecomponents, andnucleotides,but also for the production of all classes of biologicalmacromolecules,and ofacetyl-coenzyme A,adenosine triphosphate,nicotinamide adenine dinucleotideand other key intermediate and transactional molecules needed formetabolism.Thus, in biosynthesis, any of an array ofcompounds,from simple to complex, are converted into other compounds, and so it includes both thecatabolismandanabolism(building up and breaking down) of complex molecules (includingmacromolecules). Biosynthetic processes are often represented via charts ofmetabolic pathways.A particular biosynthetic pathway may be located within a single cellularorganelle(e.g., mitochondrial fatty acid synthesis pathways), while others involve enzymes that are located across an array of cellular organelles and structures (e.g., the biosynthesis of glycosylated cell surface proteins).
Elements of biosynthesis
[edit]Elements of biosynthesis include:precursorcompounds,chemical energy(e.g.ATP), and catalytic enzymes which may needcoenzymes(e.g.NADH,NADPH). These elements createmonomers,the building blocks for macromolecules. Some important biological macromolecules include:proteins,which are composed ofamino acidmonomers joined viapeptide bonds,andDNAmolecules, which are composed of nucleotides joined viaphosphodiester bonds.
Properties of chemical reactions
[edit]Biosynthesis occurs due to a series of chemical reactions. For these reactions to take place, the following elements are necessary:[1]
- Precursor compounds:these compounds are the starting molecules orsubstratesin a reaction. These may also be viewed as thereactantsin a given chemical process.
- Chemical energy:chemical energy can be found in the form of high energy molecules. These molecules are required for energetically unfavourable reactions. Furthermore, thehydrolysisof these compounds drives a reaction forward. High energy molecules, such asATP,have threephosphates.Often, the terminal phosphate is split off during hydrolysis and transferred to another molecule.
- Catalysts:these may be for examplemetal ionsorcoenzymesand they catalyze a reaction by increasing therate of the reactionand lowering theactivation energy.
In the simplest sense, the reactions that occur in biosynthesis have the following format:[2]
Some variations of this basic equation which will be discussed later in more detail are:[3]
- Simple compounds which are converted into other compounds, usually as part of a multiple step reaction pathway. Two examples of this type of reaction occur during the formation ofnucleic acidsand thechargingoftRNAprior totranslation.For some of these steps, chemical energy is required:
- Simple compounds that are converted into other compounds with the assistance of cofactors. For example, the synthesis ofphospholipidsrequires acetyl CoA, while the synthesis of another membrane component,sphingolipids,requires NADH and FADH for the formation thesphingosinebackbone. The general equation for these examples is:
- Simple compounds that join to create a macromolecule. For example,fatty acidsjoin to form phospholipids. In turn, phospholipids andcholesterolinteractnoncovalentlyin order to form thelipid bilayer.This reaction may be depicted as follows:
Lipid
[edit]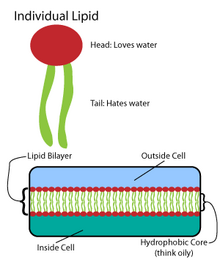
Many intricate macromolecules are synthesized in a pattern of simple, repeated structures.[4]For example, the simplest structures of lipids arefatty acids.Fatty acids arehydrocarbonderivatives; they contain acarboxyl group"head" and a hydrocarbon chain "tail".[4]These fatty acids create larger components, which in turn incorporate noncovalent interactions to form the lipid bilayer.[4] Fatty acid chains are found in two major components of membrane lipids:phospholipidsandsphingolipids.A third major membrane component,cholesterol,does not contain these fatty acid units.[5]
Eukaryotic phospholipids
[edit]The foundation of allbiomembranesconsists of abilayerstructure of phospholipids.[6]The phospholipid molecule isamphipathic;it contains ahydrophilicpolar head and ahydrophobicnonpolar tail.[4]The phospholipid heads interact with each other and aqueous media, while the hydrocarbon tails orient themselves in the center, away from water.[7]These latter interactions drive the bilayer structure that acts as a barrier for ions and molecules.[8]
There are various types of phospholipids; consequently, their synthesis pathways differ. However, the first step in phospholipid synthesis involves the formation ofphosphatidateordiacylglycerol 3-phosphateat theendoplasmic reticulumandouter mitochondrial membrane.[7]The synthesis pathway is found below:

The pathway starts with glycerol 3-phosphate, which gets converted tolysophosphatidatevia the addition of a fatty acid chain provided byacyl coenzyme A.[9]Then, lysophosphatidate is converted to phosphatidate via the addition of another fatty acid chain contributed by a second acyl CoA; all of these steps are catalyzed by the glycerol phosphateacyltransferaseenzyme.[9]Phospholipid synthesis continues in the endoplasmic reticulum, and the biosynthesis pathway diverges depending on the components of the particular phospholipid.[9]
Sphingolipids
[edit]Like phospholipids, these fatty acid derivatives have a polar head and nonpolar tails.[5]Unlike phospholipids, sphingolipids have asphingosinebackbone.[10]Sphingolipids exist ineukaryoticcells and are particularly abundant in thecentral nervous system.[7]For example,sphingomyelinis part of themyelin sheathof nerve fibers.[11]
Sphingolipids are formed fromceramidesthat consist of a fatty acid chain attached to the amino group of a sphingosine backbone. These ceramides are synthesized from theacylationof sphingosine.[11]The biosynthetic pathway for sphingosine is found below:

As the image denotes, during sphingosine synthesis,palmitoyl CoAandserineundergo acondensation reactionwhich results in the formation of 3-dehydrosphinganine.[7]This product is then reduced to form dihydrospingosine, which is converted to sphingosine via theoxidation reactionbyFAD.[7]
Cholesterol
[edit]Thislipidbelongs to a class of molecules calledsterols.[5]Sterols have four fused rings and ahydroxyl group.[5]Cholesterol is a particularly important molecule. Not only does it serve as a component of lipid membranes, it is also a precursor to severalsteroidhormones, includingcortisol,testosterone,andestrogen.[12]
Cholesterol is synthesized fromacetyl CoA.[12]The pathway is shown below:

More generally, this synthesis occurs in three stages, with the first stage taking place in thecytoplasmand the second and third stages occurring in the endoplasmic reticulum.[9]The stages are as follows:[12]
- 1. The synthesis ofisopentenyl pyrophosphate,the "building block" of cholesterol
- 2. The formation ofsqualenevia the condensation of six molecules of isopentenyl phosphate
- 3. The conversion of squalene into cholesterol via several enzymatic reactions
Nucleotides
[edit]The biosynthesis ofnucleotidesinvolves enzyme-catalyzedreactions that convert substrates into more complex products.[1]Nucleotides are the building blocks ofDNAandRNA.Nucleotides are composed of a five-membered ring formed fromribosesugar in RNA, anddeoxyribosesugar in DNA; these sugars are linked to apurineorpyrimidinebase with aglycosidic bondand aphosphategroup at the5' locationof the sugar.[13]
Purine nucleotides
[edit]
The DNA nucleotidesadenosineandguanosineconsist of a purine base attached to a ribose sugar with a glycosidic bond. In the case of RNA nucleotidesdeoxyadenosineanddeoxyguanosine,the purine bases are attached to a deoxyribose sugar with a glycosidic bond. The purine bases on DNA and RNA nucleotides are synthesized in a twelve-step reaction mechanism present in most single-celled organisms. Highereukaryotesemploy a similarreaction mechanismin ten reaction steps. Purine bases are synthesized by convertingphosphoribosyl pyrophosphate(PRPP) toinosine monophosphate(IMP), which is the first key intermediate in purine base biosynthesis.[14]Further enzymatic modification ofIMPproduces the adenosine and guanosine bases of nucleotides.
- The first step in purine biosynthesis is acondensation reaction,performed byglutamine-PRPP amidotransferase.This enzyme transfers theamino groupfromglutamineto PRPP, forming5-phosphoribosylamine.The following step requires the activation ofglycineby the addition of aphosphategroup fromATP.
- GAR synthetase[15]performs the condensation of activated glycine onto PRPP, formingglycineamide ribonucleotide(GAR).
- GAR transformylaseadds aformyl grouponto the amino group of GAR, forming formylglycinamide ribonucleotide (FGAR).
- FGAR amidotransferase[16]catalyzes the addition of a nitrogen group to FGAR, forming formylglycinamidine ribonucleotide (FGAM).
- FGAM cyclasecatalyzes ring closure, which involves removal of a water molecule, forming the 5-memberedimidazolering5-aminoimidazole ribonucleotide(AIR).
- N5-CAIR synthetase transfers acarboxylgroup, forming the intermediate N5-carboxyaminoimidazole ribonucleotide (N5-CAIR).[17]
- N5-CAIR mutaserearranges the carboxyl functional group and transfers it onto the imidazole ring, formingcarboxyamino- imidazole ribonucleotide(CAIR). The two step mechanism of CAIR formation from AIR is mostly found in single celled organisms. Higher eukaryotes contain the enzyme AIR carboxylase,[18]which transfers a carboxyl group directly to AIR imidazole ring, forming CAIR.
- SAICAR synthetaseforms apeptide bondbetweenaspartateand the added carboxyl group of the imidazole ring, formingN-succinyl-5-aminoimidazole-4-carboxamide ribonucleotide(SAICAR).
- SAICAR lyaseremoves the carbon skeleton of the added aspartate, leaving the amino group and forming5-aminoimidazole-4-carboxamide ribonucleotide(AICAR).
- AICAR transformylasetransfers a carbonyl group to AICAR, formingN-formylaminoimidazole- 4-carboxamide ribonucleotide(FAICAR).
- The final step involves the enzymeIMP synthase,which performs the purine ring closure and forms the inosine monophosphate intermediate.[5]
Pyrimidine nucleotides
[edit]
Other DNA and RNA nucleotide bases that are linked to the ribose sugar via a glycosidic bond arethymine,cytosineanduracil(which is only found in RNA). Uridine monophosphatebiosynthesis involves an enzyme that is located in themitochondrial inner membraneand multifunctional enzymes that are located in thecytosol.[19]
- The first step involves the enzymecarbamoyl phosphate synthasecombiningglutaminewithCO2in an ATP dependent reaction to formcarbamoyl phosphate.
- Aspartate carbamoyltransferasecondensescarbamoyl phosphate with aspartate to form uridosuccinate.
- Dihydroorotaseperformsring closure,a reaction that loses water, to formdihydroorotate.
- Dihydroorotate dehydrogenase,located within the mitochondrial inner membrane,[19]oxidizes dihydroorotate toorotate.
- Orotate phosphoribosyl hydrolase (OMP pyrophosphorylase) condenses orotate withPRPPto formorotidine-5'-phosphate.
- OMP decarboxylasecatalyzes the conversion of orotidine-5'-phosphate toUMP.[20]
After the uridine nucleotide base is synthesized, the other bases, cytosine and thymine are synthesized. Cytosine biosynthesis is a two-step reaction which involves the conversion of UMP toUTP.Phosphateaddition to UMP is catalyzed by akinaseenzyme. The enzymeCTP synthasecatalyzes the next reaction step: the conversion of UTP toCTPby transferring anamino groupfrom glutamine to uridine; this forms the cytosine base of CTP.[21]The mechanism, which depicts the reaction UTP + ATP + glutamine ⇔ CTP + ADP + glutamate, is below:


Cytosine is a nucleotide that is present in both DNA and RNA. However, uracil is only found in RNA. Therefore, after UTP is synthesized, it is must be converted into adeoxyform to be incorporated into DNA. This conversion involves the enzymeribonucleoside triphosphate reductase.This reaction that removes the 2'-OH of the ribose sugar to generate deoxyribose is not affected by the bases attached to the sugar. This non-specificity allows ribonucleoside triphosphate reductase to convert allnucleotide triphosphatestodeoxyribonucleotideby a similar mechanism.[21]
In contrast to uracil, thymine bases are found mostly in DNA, not RNA. Cells do not normally contain thymine bases that are linked to ribose sugars in RNA, thus indicating that cells only synthesize deoxyribose-linked thymine. The enzymethymidylate synthetaseis responsible for synthesizing thymine residues fromdUMPtodTMP.This reaction transfers amethylgroup onto the uracil base of dUMP to generate dTMP.[21]The thymidylate synthase reaction, dUMP + 5,10-methylenetetrahydrofolate ⇔ dTMP + dihydrofolate, is shown to the right.
DNA
[edit]
Although there are differences betweeneukaryoticandprokaryoticDNA synthesis, the following section denotes key characteristics of DNA replication shared by both organisms.
DNAis composed ofnucleotidesthat are joined byphosphodiester bonds.[4]DNA synthesis,which takes place in thenucleus,is asemiconservativeprocess, which means that the resulting DNA molecule contains an original strand from the parent structure and a new strand.[22]DNA synthesis is catalyzed by a family ofDNA polymerasesthat require four deoxynucleoside triphosphates, atemplate strand,and aprimerwith a free 3'OH in which to incorporate nucleotides.[23]
In order for DNA replication to occur, areplication forkis created by enzymes calledhelicaseswhich unwind the DNA helix.[23]Topoisomerasesat the replication fork removesupercoilscaused by DNA unwinding, andsingle-stranded DNA binding proteinsmaintain the two single-stranded DNA templates stabilized prior to replication.[13]
DNA synthesis is initiated by theRNA polymeraseprimase,which makes an RNA primer with a free 3'OH.[23]This primer is attached to the single-stranded DNA template, and DNA polymerase elongates the chain by incorporating nucleotides; DNA polymerase also proofreads the newly synthesized DNA strand.[23]
During the polymerization reaction catalyzed by DNA polymerase, anucleophilic attackoccurs by the 3'OH of the growing chain on the innermost phosphorus atom of a deoxynucleoside triphosphate; this yields the formation of aphosphodiester bridgethat attaches a new nucleotide and releasespyrophosphate.[9]
Two types of strands are created simultaneously during replication: theleading strand,which is synthesized continuously and grows towards the replication fork, and thelagging strand,which is made discontinuously inOkazaki fragmentsand grows away from the replication fork.[22]Okazaki fragments arecovalentlyjoined byDNA ligaseto form a continuous strand.[22] Then, to complete DNA replication, RNA primers are removed, and the resulting gaps are replaced with DNA and joined via DNA ligase.[22]
Amino acids
[edit]A protein is a polymer that is composed fromamino acidsthat are linked bypeptide bonds.There are more than300 amino acidsfound in nature of which only twenty two, known as theproteinogenic amino acids,are the building blocks for protein.[24]Onlygreen plantsand mostmicrobesare able tosynthesizeall of the 20 standard amino acids that are needed by all living species.Mammalscan only synthesize ten of the twenty standard amino acids. The other amino acids,valine,methionine,leucine,isoleucine,phenylalanine,lysine,threonineandtryptophanfor adults andhistidine,andargininefor babies are obtained through diet.[25]
Amino acid basic structure
[edit]
The general structure of the standard amino acids includes aprimary amino group,acarboxyl groupand thefunctional groupattached to theα-carbon.The different amino acids are identified by the functional group. As a result of the three different groups attached to the α-carbon, amino acids areasymmetrical molecules.For all standard amino acids, exceptglycine,the α-carbon is achiral center.In the case of glycine, the α-carbon has two hydrogen atoms, thus adding symmetry to this molecule. With the exception ofproline,all of the amino acids found in life have theL-isoformconformation. Proline has a functional group on the α-carbon that forms a ring with the amino group.[24]

Nitrogen source
[edit]One major step in amino acid biosynthesis involves incorporating a nitrogen group onto the α-carbon. In cells, there are two major pathways of incorporating nitrogen groups. One pathway involves the enzymeglutamine oxoglutarate aminotransferase(GOGAT) which removes theamideamino group ofglutamineand transfers it onto2-oxoglutarate,producing twoglutamatemolecules. In this catalysis reaction, glutamine serves as the nitrogen source. An image illustrating this reaction is found to the right.
The other pathway for incorporating nitrogen onto the α-carbon of amino acids involves the enzymeglutamate dehydrogenase(GDH). GDH is able to transferammoniaonto 2-oxoglutarate and form glutamate. Furthermore, the enzymeglutamine synthetase(GS) is able to transfer ammonia onto glutamate and synthesize glutamine, replenishing glutamine.[26]
The glutamate family of amino acids
[edit]Theglutamatefamily of amino acids includes the amino acids that derive from the amino acid glutamate. This family includes: glutamate,glutamine,proline,andarginine.This family also includes the amino acidlysine,which is derived fromα-ketoglutarate.[27]
The biosynthesis of glutamate and glutamine is a key step in the nitrogen assimilation discussed above. The enzymesGOGATandGDHcatalyze thenitrogen assimilationreactions.
In bacteria, the enzymeglutamate 5-kinaseinitiates the biosynthesis of proline by transferring a phosphate group from ATP onto glutamate. The next reaction is catalyzed by the enzymepyrroline-5-carboxylate synthase(P5CS), which catalyzes the reduction of theϒ-carboxylgroup of L-glutamate 5-phosphate. This results in the formation of glutamate semialdehyde, which spontaneously cyclizes to pyrroline-5-carboxylate. Pyrroline-5-carboxylate is further reduced by the enzyme pyrroline-5-carboxylate reductase (P5CR) to yield a proline amino acid.[28]
In the first step of arginine biosynthesis in bacteria, glutamate isacetylatedby transferring the acetyl group from acetyl-CoA at the N-α position; this prevents spontaneous cyclization. The enzymeN-acetylglutamate synthase(glutamate N-acetyltransferase) is responsible for catalyzing the acetylation step. Subsequent steps are catalyzed by the enzymesN-acetylglutamate kinase,N-acetyl-gamma-glutamyl-phosphate reductase,andacetylornithine/succinyldiamino pimelate aminotransferaseand yield the N-acetyl-L-ornithine. The acetyl group of acetylornithine is removed by the enzymeacetylornithinase(AO) orornithine acetyltransferase(OAT), and this yieldsornithine.Then, the enzymescitrullineandargininosuccinateconvert ornithine to arginine.[29]
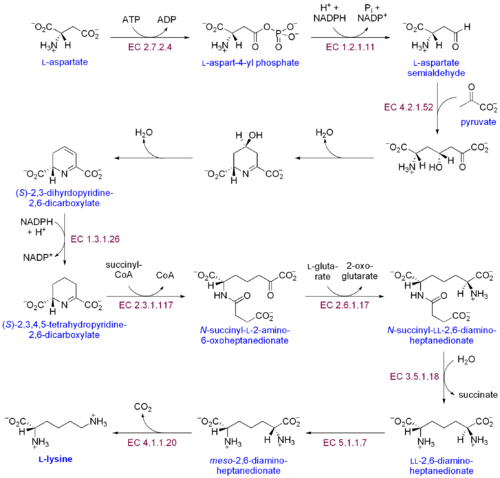
There are two distinct lysine biosynthetic pathways: the diaminopimelic acid pathway and theα-aminoadipate pathway.The most common of the two synthetic pathways is the diaminopimelic acid pathway; it consists of several enzymatic reactions that add carbon groups to aspartate to yield lysine:[30]
- Aspartate kinaseinitiates the diaminopimelic acid pathway by phosphorylating aspartate and producing aspartyl phosphate.
- Aspartate semialdehyde dehydrogenasecatalyzes theNADPH-dependent reduction of aspartyl phosphate to yield aspartate semialdehyde.
- 4-hydroxy-tetrahydrodipicolinate synthaseadds apyruvategroup to the β-aspartyl-4-semialdehyde, and a water molecule is removed. This causescyclizationand gives rise to (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate.
- 4-hydroxy-tetrahydrodipicolinate reductasecatalyzes the reduction of (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate by NADPH to yield Δ'-piperideine-2,6-dicarboxylate (2,3,4,5-tetrahydrodipicolinate) and H2O.
- Tetrahydrodipicolinate acyltransferasecatalyzes the acetylation reaction that results in ring opening and yields N-acetyl α-amino-ε-ketopimelate.
- N-succinyl-α-amino-ε-ketopimelate-glutamate aminotransaminasecatalyzes the transamination reaction that removes the keto group of N-acetyl α-amino-ε-ketopimelate and replaces it with an amino group to yield N-succinyl-L-diaminopimelate.[31]
- N-acyldiaminopimelate deacylasecatalyzes the deacylation of N-succinyl-L-diaminopimelate to yield L,L-diaminopimelate.[32]
- DAP epimerasecatalyzes the conversion of L,L-diaminopimelate to themesoform of L,L-diaminopimelate.[33]
- DAP decarboxylasecatalyzes the removal of the carboxyl group, yielding L-lysine.
The serine family of amino acids
[edit]Theserinefamily of amino acid includes: serine,cysteine,andglycine.Most microorganisms and plants obtain the sulfur for synthesizingmethioninefrom the amino acid cysteine. Furthermore, the conversion of serine to glycine provides the carbons needed for the biosynthesis of the methionine andhistidine.[27]
During serine biosynthesis,[34]the enzymephosphoglycerate dehydrogenasecatalyzes the initial reaction thatoxidizes3-phospho-D-glycerateto yield3-phosphonooxypyruvate.[35]The following reaction is catalyzed by the enzymephosphoserine aminotransferase,which transfers an amino group from glutamate onto 3-phosphonooxypyruvate to yieldL-phosphoserine.[36]The final step is catalyzed by the enzymephosphoserine phosphatase,whichdephosphorylatesL-phosphoserine to yieldL-serine.[37]
There are two known pathways for the biosynthesis of glycine. Organisms that useethanolandacetateas the major carbon source utilize theglyconeogenicpathway to synthesizeglycine.The other pathway of glycine biosynthesis is known as theglycolyticpathway. This pathway converts serine synthesized from the intermediates ofglycolysisto glycine. In the glycolytic pathway, the enzymeserine hydroxymethyltransferasecatalyzes the cleavage of serine to yield glycine and transfers the cleaved carbon group of serine ontotetrahydrofolate,forming5,10-methylene-tetrahydrofolate.[38]
Cysteine biosynthesis is a two-step reaction that involves the incorporation of inorganicsulfur.In microorganisms and plants, the enzymeserine acetyltransferasecatalyzes the transfer of acetyl group fromacetyl-CoAonto L-serine to yieldO-acetyl-L-serine.[39]The following reaction step, catalyzed by the enzymeO-acetyl serine (thiol) lyase,replaces the acetyl group of O-acetyl-L-serine with sulfide to yield cysteine.[40]
The aspartate family of amino acids
[edit]Theaspartatefamily of amino acids includes:threonine,lysine,methionine,isoleucine,and aspartate. Lysine and isoleucine are considered part of the aspartate family even though part of their carbon skeleton is derived frompyruvate.In the case of methionine, the methyl carbon is derived from serine and the sulfur group, but in most organisms, it is derived from cysteine.[27]
The biosynthesis of aspartate is a one step reaction that is catalyzed by a single enzyme. The enzymeaspartate aminotransferasecatalyzes the transfer of an amino group from aspartate ontoα-ketoglutarateto yield glutamate andoxaloacetate.[41]Asparagine is synthesized by an ATP-dependent addition of an amino group onto aspartate;asparagine synthetasecatalyzes the addition of nitrogen from glutamine or soluble ammonia to aspartate to yield asparagine.[42]

The diaminopimelic acid biosynthetic pathway of lysine belongs to the aspartate family of amino acids. This pathway involves nine enzyme-catalyzed reactions that convert aspartate to lysine.[43]
- Aspartate kinasecatalyzes the initial step in the diaminopimelic acid pathway by transferring aphosphorylfrom ATP onto the carboxylate group of aspartate, which yields aspartyl-β-phosphate.[44]
- Aspartate-semialdehyde dehydrogenasecatalyzes the reduction reaction bydephosphorylationof aspartyl-β-phosphate to yield aspartate-β-semialdehyde.[45]
- Dihydrodipicolinate synthasecatalyzes thecondensationreaction of aspartate-β-semialdehyde with pyruvate to yield dihydrodipicolinic acid.[46]
- 4-hydroxy-tetrahydrodipicolinate reductasecatalyzes the reduction of dihydrodipicolinic acid to yield tetrahydrodipicolinic acid.[47]
- Tetrahydrodipicolinate N-succinyltransferasecatalyzes the transfer of a succinyl group from succinyl-CoA on to tetrahydrodipicolinic acid to yield N-succinyl-L-2,6-diaminoheptanedioate.[48]
- N-succinyldiaminopimelate aminotransferase catalyzes the transfer of an amino group from glutamate onto N-succinyl-L-2,6-diaminoheptanedioate to yield N-succinyl-L,L-diaminopimelic acid.[49]
- Succinyl-diaminopimelate desuccinylasecatalyzes the removal of acyl group from N-succinyl-L,L-diaminopimelic acid to yield L,L-diaminopimelic acid.[50]
- Diaminopimelate epimerasecatalyzes the inversion of the α-carbon of L,L-diaminopimelic acid to yieldmeso-diaminopimelic acid.[51]
- Siaminopimelate decarboxylase catalyzes the final step in lysine biosynthesis that removes the carbon dioxide group from meso-diaminopimelic acid to yield L-lysine.[52]
Proteins
[edit]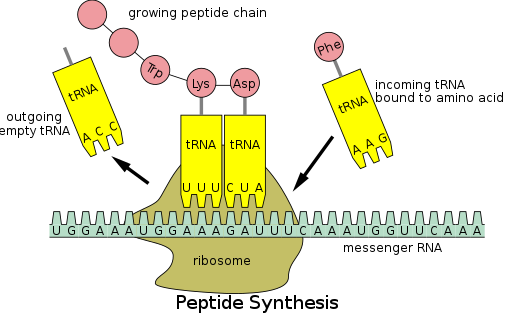
Protein synthesis occurs via a process calledtranslation.[53]During translation, genetic material calledmRNAis read byribosomesto generate a proteinpolypeptidechain.[53]This process requirestransfer RNA(tRNA) which serves as an adaptor by bindingamino acidson one end and interacting with mRNA at the other end; the latter pairing between the tRNA and mRNA ensures that the correct amino acid is added to the chain.[53]Protein synthesis occurs in three phases: initiation, elongation, and termination.[13]Prokaryotic (archaealandbacterial) translation differs fromeukaryotic translation;however, this section will mostly focus on the commonalities between the two organisms.
Additional background
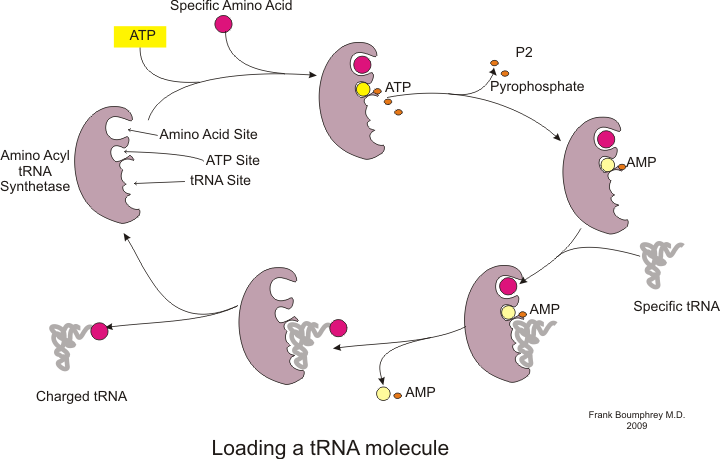
[edit]Before translation can begin, the process of binding a specific amino acid to its corresponding tRNA must occur. This reaction, called tRNA charging, is catalyzed byaminoacyl tRNA synthetase.[54]A specific tRNA synthetase is responsible for recognizing and charging a particular amino acid.[54]Furthermore, this enzyme has special discriminator regions to ensure the correct binding between tRNA and its cognate amino acid.[54]The first step for joining an amino acid to its corresponding tRNA is the formation of aminoacyl-AMP:[54]
This is followed by the transfer of the aminoacyl group from aminoacyl-AMP to a tRNA molecule. The resulting molecule isaminoacyl-tRNA:[54]
The combination of these two steps, both of which are catalyzed by aminoacyl tRNA synthetase, produces a charged tRNA that is ready to add amino acids to the growing polypeptide chain.
In addition to binding an amino acid, tRNA has a three nucleotide unit called ananticodonthatbase pairswith specific nucleotide triplets on the mRNA calledcodons;codons encode a specific amino acid.[55]This interaction is possible thanks to the ribosome, which serves as the site for protein synthesis. The ribosome possesses three tRNA binding sites: the aminoacyl site (A site), the peptidyl site (P site), and the exit site (E site).[56]
There are numerous codons within an mRNA transcript, and it is very common for an amino acid to be specified by more than one codon; this phenomenon is calleddegeneracy.[57]In all, there are 64 codons, 61 of each code for one of the 20 amino acids, while the remaining codons specify chain termination.[57]
Translation in steps
[edit]As previously mentioned, translation occurs in three phases: initiation, elongation, and termination.

Step 1: Initiation
[edit]The completion of the initiation phase is dependent on the following three events:[13]
1. The recruitment of the ribosome to mRNA
2. The binding of a charged initiator tRNA into the P site of the ribosome
3. The proper alignment of the ribosome with mRNA's start codon
Step 2: Elongation
[edit]Following initiation, the polypeptide chain is extended via anticodon:codon interactions, with the ribosome adding amino acids to the polypeptide chain one at a time. The following steps must occur to ensure the correct addition of amino acids:[58]
1. The binding of the correct tRNA into the A site of the ribosome
2. The formation of apeptide bondbetween the tRNA in the A site and the polypeptide chain attached to the tRNA in the P site
3.Translocationor advancement of the tRNA-mRNA complex by three nucleotides
Translocation "kicks off" the tRNA at the E site and shifts the tRNA from the A site into the P site, leaving the A site free for an incoming tRNA to add another amino acid.
Step 3: Termination
[edit]The last stage of translation occurs when astop codonenters the A site.[1]Then, the following steps occur:
1. The recognition of codons byrelease factors,which causes thehydrolysisof the polypeptide chain from the tRNA located in the P site[1]
2. The release of the polypeptide chain[57]
3. The dissociation and "recycling" of the ribosome for future translation processes[57]
A summary table of the key players in translation is found below:
| Key players in Translation | Translation Stage | Purpose |
|---|---|---|
| tRNA synthetase | before initiation | Responsible for tRNA charging |
| mRNA | initiation, elongation, termination | Template for protein synthesis; contains regions named codons which encode amino acids |
| tRNA | initiation, elongation, termination | Binds ribosomes sites A, P, E; anticodon base pairs with mRNA codon to ensure that the correct amino acid is incorporated into the growing polypeptide chain |
| ribosome | initiation, elongation, termination | Directs protein synthesis and catalyzes the formation of the peptide bond |
Diseases associated with macromolecule deficiency
[edit]
Errors in biosynthetic pathways can have deleterious consequences including the malformation of macromolecules or the underproduction of functional molecules. Below are examples that illustrate the disruptions that occur due to these inefficiencies.
- Familial hypercholesterolemia:this disorder is characterized by the absence of functionalreceptorsforLDL.[59]Deficiencies in the formation of LDL receptors may cause faulty receptors which disrupt theendocyticpathway, inhibiting the entry of LDL into the liver and other cells.[59]This causes a buildup of LDL in the blood plasma, which results inatherosclerotic plaquesthat narrow arteries and increase the risk of heart attacks.[59]
- Lesch–Nyhan syndrome:this genetic disease is characterized byself- mutilation,mental deficiency, andgout.[60]It is caused by the absence ofhypoxanthine-guanine phosphoribosyltransferase,which is a necessary enzyme for purine nucleotide formation.[60]The lack of enzyme reduces the level of necessary nucleotides and causes the accumulation of biosynthesisintermediates,which results in the aforementioned unusual behavior.[60]
- Severe combined immunodeficiency (SCID):SCID is characterized by a loss ofT cells.[61]Shortage of these immune system components increases the susceptibility to infectious agents because the affected individuals cannot developimmunological memory.[61]This immunological disorder results from a deficiency inadenosine deaminaseactivity, which causes a buildup ofdATP.These dATP molecules then inhibit ribonucleotide reductase, which prevents of DNA synthesis.[61]
- Huntington's disease:thisneurologicaldisease is caused from errors that occur during DNA synthesis.[62]These errors or mutations lead to the expression of a mutanthuntingtinprotein, which contains repetitiveglutamineresidues that are encoded by expandingCAG trinucleotide repeatsin the gene.[62]Huntington's disease is characterized by neuronal loss andgliosis.Symptoms of the disease include: movement disorder,cognitivedecline, and behavioral disorder.[63]
See also
[edit]- Lipids
- Phospholipid bilayer
- Nucleotides
- DNA
- DNA replication
- Proteinogenic amino acid
- Codon table
- Prostaglandin
- Porphyrins
- Chlorophylls and bacteriochlorophylls
- Vitamin B12
References
[edit]- ^abcdAlberts, Bruce (2008).Molecular Biology of the Cell(5th ed.). New York: Garland Science.ISBN978-0815341055.
- ^Zumdahl, Steven S. Zumdahl, Susan A. (2008).Chemistry(8th ed.). CA: Cengage Learning.ISBN978-0547125329.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. (2013).Fundamentals of Biochemistry: Life at the Molecular Level(4th ed.). Hoboken, NJ: Wiley.ISBN978-0470547847.
- ^abcdeLodish, Harvey; et al. (2007).Molecular cell biology(6th ed.). New York: W.H. Freeman.ISBN978-0716743668.
- ^abcdeCox, David L. Nelson, Michael M. (2008).Lehninger principles of biochemistry(5th ed.). New York: W.H. Freeman.ISBN9780716771081.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Hanin, Israel (2013).Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations.Springer.ISBN978-1475713664.
- ^abcdeVance, Dennis E.; Vance, Jean E. (2008).Biochemistry of lipids, lipoproteins and membranes(5th ed.). Amsterdam: Elsevier.ISBN978-0444532190.
- ^Katsaras, J.; et al. (2001).Lipid bilayers: structure and interactions; with 6 tables.Berlin [u.a.]: Springer.ISBN978-3540675556.
- ^abcdeStryer, Jeremy M. Berg; John L. Tymoczko; Lubert (2007).Biochemistry(6. ed., 3. print. ed.). New York: Freeman.ISBN978-0716787242.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Gault, CR; LM Obeid; YA Hannun (2010). "An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown".Sphingolipids as Signaling and Regulatory Molecules.Advances in Experimental Medicine and Biology. Vol. 688. pp. 1–23.doi:10.1007/978-1-4419-6741-1_1.ISBN978-1-4419-6740-4.PMC3069696.PMID20919643.
- ^abSiegel, George J. (1999).Basic neurochemistry: molecular, cellular and medical aspects(6. ed.). Philadelphia, Pa. [u.a.]: Lippincott Williams & Wilkins.ISBN978-0397518203.
- ^abcHarris, J. Robin (2010).Cholesterol binding and cholesterol transport proteins: structure and function in health and disease.Dordrecht: Springer.ISBN978-9048186211.
- ^abcdWatson, James D.; et al. (2007).Molecular biology of the gene(6th ed.). San Francisco, Calif.: Benjamin Cummings.ISBN978-0805395921.
- ^Kappock, TJ; Ealick, SE; Stubbe, J (October 2000). "Modular evolution of the purine biosynthetic pathway".Current Opinion in Chemical Biology.4(5): 567–72.doi:10.1016/s1367-5931(00)00133-2.PMID11006546.
- ^Sampei, G; Baba, S; Kanagawa, M; Yanai, H; Ishii, T; Kawai, H; Fukai, Y; Ebihara, A; Nakagawa, N; Kawai, G (October 2010). "Crystal structures of glycinamide ribonucleotide synthetase, PurD, from thermophilic eubacteria".Journal of Biochemistry.148(4): 429–38.doi:10.1093/jb/mvq088.PMID20716513.
- ^Hoskins, AA; Anand, R; Ealick, SE; Stubbe, J (Aug 17, 2004). "The formylglycinamide ribonucleotide amidotransferase complex from Bacillus subtilis: metabolite-mediated complex formation".Biochemistry.43(32): 10314–27.doi:10.1021/bi049127h.PMID15301530.
- ^Mueller, EJ; Meyer, E; Rudolph, J; Davisson, VJ; Stubbe, J (Mar 1, 1994). "N5-carboxyaminoimidazole ribonucleotide: evidence for a new intermediate and two new enzymatic activities in the de novo purine biosynthetic pathway of Escherichia coli".Biochemistry.33(8): 2269–78.doi:10.1021/bi00174a038.PMID8117684.
- ^Firestine, SM; Poon, SW; Mueller, EJ; Stubbe, J; Davisson, VJ (Oct 4, 1994). "Reactions catalyzed by 5-aminoimidazole ribonucleotide carboxylases from Escherichia coli and Gallus gallus: a case for divergent catalytic mechanisms".Biochemistry.33(39): 11927–34.doi:10.1021/bi00205a031.PMID7918411.
- ^abSrere, PA (1987). "Complexes of sequential metabolic enzymes".Annual Review of Biochemistry.56(1): 89–124.doi:10.1146/annurev.bi.56.070187.000513.PMID2441660.
- ^Broach, edited by Jeffrey N. Strathern, Elizabeth W. Jones, James R. (1981).The Molecular biology of the yeast Saccharomyces.Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory.ISBN978-0879691394.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^abcO'Donovan, GA; Neuhard, J (September 1970)."Pyrimidine metabolism in microorganisms".Bacteriological Reviews.34(3): 278–343.doi:10.1128/MMBR.34.3.278-343.1970.PMC378357.PMID4919542.
- ^abcdGeer, Gerald Karp; responsible for the revision of chapter 15 Peter van der (2004).Cell and molecular biology: concepts and experiments(4th ed., Wiley International ed.). New York: J. Wiley & Sons.ISBN978-0471656654.
{{cite book}}:CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^abcdGriffiths, Anthony J. F. (1999).Modern genetic analysis(2. print. ed.). New York: Freeman.ISBN978-0716731184.
- ^abWu, G (May 2009). "Amino acids: metabolism, functions, and nutrition".Amino Acids.37(1): 1–17.doi:10.1007/s00726-009-0269-0.PMID19301095.S2CID1870305.
- ^Mousdale, D. M.; Coggins, J. R. (1991). "Amino Acid Synthesis".Target Sites for Herbicide Action.pp. 29–56.doi:10.1007/978-1-4899-2433-9_2.ISBN978-1-4899-2435-3.
- ^Miflin, B. J.; Lea, P. J. (1977). "Amino Acid Metabolism".Annual Review of Plant Physiology.28:299–329.doi:10.1146/annurev.pp.28.060177.001503.
- ^abcUmbarger, HE (1978). "Amino acid biosynthesis and its regulation".Annual Review of Biochemistry.47(1): 532–606.doi:10.1146/annurev.bi.47.070178.002533.PMID354503.
- ^Pérez-Arellano, I; Carmona-Alvarez, F; Martínez, AI; Rodríguez-Díaz, J; Cervera, J (March 2010)."Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease".Protein Science.19(3): 372–82.doi:10.1002/pro.340.PMC2866264.PMID20091669.
- ^Xu, Y; Labedan, B; Glansdorff, N (March 2007)."Surprising arginine biosynthesis: a reappraisal of the enzymology and evolution of the pathway in microorganisms".Microbiology and Molecular Biology Reviews.71(1): 36–47.doi:10.1128/MMBR.00032-06.PMC1847373.PMID17347518.
- ^"MetaCyc: L-lysine biosynthesis I".
- ^PETERKOFSKY, B; GILVARG, C (May 1961)."N-Succinyl-L-diaminopimelic-glutamic transaminase".The Journal of Biological Chemistry.236(5): 1432–8.doi:10.1016/S0021-9258(18)64192-4.PMID13734750.
- ^KINDLER, SH; GILVARG, C (December 1960)."N-Succinyl-L-2,6-diaminopimelic acid deacylase".The Journal of Biological Chemistry.235:3532–5.doi:10.1016/S0021-9258(18)64502-8.PMID13756049.
- ^Born, TL; Blanchard, JS (October 1999). "Structure/function studies on enzymes in the diaminopimelate pathway of bacterial cell wall biosynthesis".Current Opinion in Chemical Biology.3(5): 607–13.doi:10.1016/s1367-5931(99)00016-2.PMID10508663.
- ^"Escherichia coli K-12 substr. MG1655".serine biosynthesis.SRI International.Retrieved12 December2013.
- ^Bell, JK; Grant, GA; Banaszak, LJ (Mar 30, 2004). "Multiconformational states in phosphoglycerate dehydrogenase".Biochemistry.43(12): 3450–8.doi:10.1021/bi035462e.PMID15035616.
- ^Dubnovitsky, AP; Kapetaniou, EG; Papageorgiou, AC (January 2005)."Enzyme adaptation to alkaline pH: atomic resolution (1.08 A) structure of phosphoserine aminotransferase from Bacillus alcalophilus".Protein Science.14(1): 97–110.doi:10.1110/ps.041029805.PMC2253317.PMID15608117.
- ^Wang, W; Kim, R; Jancarik, J; Yokota, H; Kim, SH (Jan 10, 2001)."Crystal structure of phosphoserine phosphatase from Methanococcus jannaschii, a hyperthermophile, at 1.8 A resolution".Structure.9(1): 65–71.doi:10.1016/s0969-2126(00)00558-x.PMID11342136.
- ^Monschau, N; Stahmann, KP; Sahm, H; McNeil, JB; Bognar, AL (May 1, 1997)."Identification of Saccharomyces cerevisiae GLY1 as a threonine aldolase: a key enzyme in glycine biosynthesis".FEMS Microbiology Letters.150(1): 55–60.doi:10.1111/j.1574-6968.1997.tb10349.x.PMID9163906.
- ^Pye, VE; Tingey, AP; Robson, RL; Moody, PC (Sep 24, 2004)."The structure and mechanism of serine acetyltransferase from Escherichia coli".The Journal of Biological Chemistry.279(39): 40729–36.doi:10.1074/jbc.M403751200.PMID15231846.
- ^Huang, B; Vetting, MW; Roderick, SL (May 2005)."The active site of O-acetylserine sulfhydrylase is the anchor point for bienzyme complex formation with serine acetyltransferase".Journal of Bacteriology.187(9): 3201–5.doi:10.1128/JB.187.9.3201-3205.2005.PMC1082839.PMID15838047.
- ^McPhalen, CA; Vincent, MG; Picot, D;Jansonius, JN;Lesk, AM; Chothia, C (Sep 5, 1992). "Domain closure in mitochondrial aspartate aminotransferase".Journal of Molecular Biology.227(1): 197–213.doi:10.1016/0022-2836(92)90691-C.PMID1522585.
- ^Larsen, TM; Boehlein, SK; Schuster, SM; Richards, NG; Thoden, JB; Holden, HM; Rayment, I (Dec 7, 1999). "Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product".Biochemistry.38(49): 16146–57.CiteSeerX10.1.1.453.5998.doi:10.1021/bi9915768.PMID10587437.
- ^Velasco, AM; Leguina, JI; Lazcano, A (October 2002). "Molecular evolution of the lysine biosynthetic pathways".Journal of Molecular Evolution.55(4): 445–59.Bibcode:2002JMolE..55..445V.doi:10.1007/s00239-002-2340-2.PMID12355264.S2CID19460256.
- ^Kotaka, M; Ren, J; Lockyer, M; Hawkins, AR; Stammers, DK (Oct 20, 2006)."Structures of R- and T-state Escherichia coli aspartokinase III. Mechanisms of the allosteric transition and inhibition by lysine".The Journal of Biological Chemistry.281(42): 31544–52.doi:10.1074/jbc.M605886200.PMID16905770.
- ^Hadfield, A; Kryger, G; Ouyang, J; Petsko, GA; Ringe, D; Viola, R (Jun 18, 1999). "Structure of aspartate-beta-semialdehyde dehydrogenase from Escherichia coli, a key enzyme in the aspartate family of amino acid biosynthesis".Journal of Molecular Biology.289(4): 991–1002.doi:10.1006/jmbi.1999.2828.PMID10369777.
- ^Mirwaldt, C; Korndörfer, I; Huber, R (Feb 10, 1995). "The crystal structure of dihydrodipicolinate synthase from Escherichia coli at 2.5 A resolution".Journal of Molecular Biology.246(1): 227–39.doi:10.1006/jmbi.1994.0078.PMID7853400.
- ^Cirilli, M; Zheng, R; Scapin, G; Blanchard, JS (Sep 16, 2003). "The three-dimensional structures of the Mycobacterium tuberculosis dihydrodipicolinate reductase-NADH-2,6-PDC and -NADPH-2,6-PDC complexes. Structural and mutagenic analysis of relaxed nucleotide specificity".Biochemistry.42(36): 10644–50.doi:10.1021/bi030044v.PMID12962488.
- ^Beaman, TW; Binder, DA; Blanchard, JS; Roderick, SL (Jan 21, 1997). "Three-dimensional structure of tetrahydrodipicolinate N-succinyltransferase".Biochemistry.36(3): 489–94.doi:10.1021/bi962522q.PMID9012664.
- ^Weyand, S; Kefala, G; Weiss, MS (Mar 30, 2007). "The three-dimensional structure of N-succinyldiaminopimelate aminotransferase from Mycobacterium tuberculosis".Journal of Molecular Biology.367(3): 825–38.doi:10.1016/j.jmb.2007.01.023.PMID17292400.
- ^Nocek, BP; Gillner, DM; Fan, Y; Holz, RC; Joachimiak, A (Apr 2, 2010)."Structural basis for catalysis by the mono- and dimetalated forms of the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase".Journal of Molecular Biology.397(3): 617–26.doi:10.1016/j.jmb.2010.01.062.PMC2885003.PMID20138056.
- ^Pillai, B; Cherney, M; Diaper, CM; Sutherland, A; Blanchard, JS; Vederas, JC; James, MN (Nov 23, 2007). "Dynamics of catalysis revealed from the crystal structures of mutants of diaminopimelate epimerase".Biochemical and Biophysical Research Communications.363(3): 547–53.doi:10.1016/j.bbrc.2007.09.012.PMID17889830.
- ^Gokulan, K; Rupp, B; Pavelka MS, Jr; Jacobs WR, Jr; Sacchettini, JC (May 16, 2003)."Crystal structure of Mycobacterium tuberculosis diaminopimelate decarboxylase, an essential enzyme in bacterial lysine biosynthesis".The Journal of Biological Chemistry.278(20): 18588–96.doi:10.1074/jbc.M301549200.PMID12637582.
- ^abcWeaver, Robert F. (2005).Molecular biology(3rd ed.). Boston: McGraw-Hill Higher Education.ISBN978-0-07-284611-9.
- ^abcdeCooper, Geoffrey M. (2000).The Cell: A Molecular Approach(2nd ed.). Washington (DC): ASM Press.ISBN978-0878931064.
- ^Jackson, R.J.; et al. (February 2010)."The mechanism of eukaryotic translation initiation and principles of its regulation".Molecular Cell Biology.10(2): 113–127.doi:10.1038/nrm2838.PMC4461372.PMID20094052.
- ^Green, Rachel; Harry F. Noller; et al. (1997). "Ribosomes and Translation".Annu. Rev. Biochem.66:679–716.doi:10.1146/annurev.biochem.66.1.679.PMID9242921.
- ^abcdWeissbach, Herbert; Pestka, Sidney (1977).Molecular Mechanisms of Protein Biosynthesis.New York: Academic Press.ISBN978-0127442501.
- ^Frank, J; Haixiao Gao; et al. (September 2007)."The process of mRNA–tRNA translocation".PNAS.104(50): 19671–19678.doi:10.1073/pnas.0708517104.PMC2148355.PMID18003906.
- ^abcBandeali, Salman J.; Daye, Jad; Virani, Salim S. (30 November 2013). "Novel Therapies for Treating Familial Hypercholesterolemia".Current Atherosclerosis Reports.16(1): 382.doi:10.1007/s11883-013-0382-0.PMID24293346.S2CID8903481.
- ^abcKang, Tae Hyuk; Park, Yongjin; Bader, Joel S.; Friedmann, Theodore; Cooney, Austin John (9 October 2013)."The Housekeeping Gene Hypoxanthine Guanine Phosphoribosyltransferase (HPRT) Regulates Multiple Developmental and Metabolic Pathways of Murine Embryonic Stem Cell Neuronal Differentiation".PLOS ONE.8(10): e74967.Bibcode:2013PLoSO...874967K.doi:10.1371/journal.pone.0074967.PMC3794013.PMID24130677.
- ^abcWalport, Ken Murphy, Paul Travers, Mark (2011).Janeway's Immunobiology(8. ed.). Oxford: Taylor & Francis.ISBN978-0815342434.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^abHughes, edited by Donald C. Lo, Robert E. (2010).Neurobiology of Huntington's disease: applications to drug discovery(2nd ed.). Boca Raton: CRC Press/Taylor & Francis Group.ISBN978-0849390005.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^Biglan, Kevin M.; Ross, Christopher A.; Langbehn, Douglas R.; Aylward, Elizabeth H.; Stout, Julie C.; Queller, Sarah; Carlozzi, Noelle E.; Duff, Kevin; Beglinger, Leigh J.; Paulsen, Jane S. (26 June 2009)."Motor abnormalities in premanifest persons with Huntington's disease: The PREDICT-HD study".Movement Disorders.24(12): 1763–1772.doi:10.1002/mds.22601.PMC3048804.PMID19562761.

![{\displaystyle {\ce {Reactant ->[][enzyme] Product}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d97fbef58f64fb0b7d05d90fa0a73b05f5919fc8)

![{\displaystyle {\ce {{Precursor~molecule}+Cofactor->[][enzyme]macromolecule}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b192f25eec9ceebed179bfeb43ae27795f3a91be)


