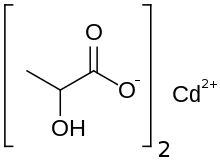
Cadmium lactate
Appearance
| |
| Names | |
|---|---|
| Other names
cadmium(2+);2-hydroxypropanoate, cadmium dilactate, bis(lactato)cadmium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.036.512 |
| EC Number |
|
PubChemCID
|
|
| RTECS number |
|
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C 6H 10CdO 6 | |
| Molar mass | 290.55 |
| Appearance | Colorless crystalls |
| Density | g/cm3 |
| Very soluble | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Cadmium lactateis an organic chemical compound, a salt ofcadmiumandlactic acidwith the formula Cd(C3H5O3)2.[1]
Synthesis
[edit]Cadmium lactate can be obtained by dissolvingcadmium carbonatein lactic acid.[2]It can also be obtained by mi xing boiling solutions of lactate of lime andcadmium sulfate.[3]
Physical properties
[edit]Cadmium lactate forms colorless (white) crystals.[4]It is soluble in water[5]but insoluble inethanol.[6][7]It is a carcinogen and poison.[8]
References
[edit]- ^Sr, Richard J. Lewis (13 June 2008).Hazardous Chemicals Desk Reference.John Wiley & Sons.p. 257.ISBN978-0-470-18024-2.Retrieved22 January2022.
- ^Watts, Henry (1865).A Dictionary of Chemistry.Longman, Green, Roberts & Green. p. 458.Retrieved22 January2022.
- ^Works of the Cavendish Society: Gmelin, Leopold. Hand-book of chemistry. 18 v. & index. 1848-72.1857. p. 489.Retrieved22 January2022.
- ^Schwartz, Mel (29 April 2002).Encyclopedia of Materials, Parts and Finishes, Second Edition.CRC Press.p. 84.ISBN978-1-4200-1716-8.Retrieved22 January2022.
- ^Armarego, W. L. F. (7 March 2003).Purification of Laboratory Chemicals.Elsevier.p. 406.ISBN978-0-08-051546-5.Retrieved22 January2022.
- ^Journal - Chemical Society, London.Chemical Society (Great Britain). 1895. p. 635.Retrieved22 January2022.
- ^Francis, William; Croft, Henry (1847).The Chemical Gazette, Or, Journal of Practical Chemistry, in All Its Applications to Pharmacy, Arts, and Manufactures.R. and J. E. Taylor. p. 487.Retrieved22 January2022.
- ^Toxic Substances.U.S. Government Printing Office.1974. p. 170.Retrieved22 January2022.
