Calcium metabolism

Calcium metabolismis the movement and regulation ofcalciumions (Ca2+)in(via thegut) andout(via the gut andkidneys) of the body, andbetweenbody compartments: theblood plasma,theextracellularandintracellularfluids, andbone.Bone acts as a calcium storage center for deposits and withdrawals as needed by the blood via continualbone remodeling.[1]: 276–277
An important aspect of calciummetabolismisplasma calciumhomeostasis,the regulation of calcium ions in theblood plasmawithinnarrow limits.[2]The level of the calcium in plasma is regulated by the hormonesparathyroid hormone(PTH) andcalcitonin.PTH is released by thechief cellsof theparathyroid glandswhen the plasma calcium level falls below the normal range in order to raise it; calcitonin is released by theparafollicular cellsof thethyroid glandwhen the plasma level of calcium is above the normal range in order to lower it.
Body compartment content[edit]
Calcium is the most abundant mineral in thehuman body.[3]The average adult body contains in total approximately 1 kg, 99% in the skeleton in the form ofcalcium phosphatesalts.[3]The extracellular fluid (ECF) contains approximately 22 mmol, of which about 9 mmol is in theplasma.[4]Approximately 10 mmol of calcium is exchanged between bone and the ECF over a period of twenty-four hours.[5]
Blood concentration[edit]
The concentration of calcium ions inside cells (in theintracellular fluid) is more than 7,000 times lower than in the blood plasma (i.e. at <0.0002 mmol/L, compared with 1.4 mmol/L in the plasma)
Normal plasma levels[edit]
The plasmatotal calciumconcentration is in the range of 2.2–2.6 mmol/L (9–10.5 mg/dL), and the normalionized calciumis 1.3–1.5 mmol/L (4.5–5.6 mg/dL).[4]The amount of total calcium in the blood varies with the level ofplasma albumin,the most abundant protein in plasma, and therefore the main carrier of protein-bound calcium in the blood. The biologic effect of calcium is, however, determined by the amount ofionized calcium,rather than the total calcium. It is therefore the plasmaionizedcalcium level which istightly regulatedto remain within very narrow limits by homeostaticnegative feedbacksystems.
Between 35 and 50% of the calcium in plasma is protein-bound, and 5–10% is in the form of complexes with organic acids and phosphates. The remainder (50–60%) is ionized. The ionized calcium can be determined directly bycolorimetry,or it can be read off fromnomograms,though the usefulness of the latter is limited when the pH and protein content of the plasma deviate widely from the normal.[4]
Function[edit]
Calcium has several main functions in the body.
Bound to serum proteins[edit]
It readily binds to proteins, particularly those with amino acids whose side chains terminate in carboxyl (-COOH) groups (e.g. glutamate residues). When such binding occurs the electrical charges on the protein chain change, causing the protein's tertiary structure (i.e. 3-dimensional form) to change. Good examples of this are several of theclotting factorsin the blood plasma, which are functionless in the absence of calcium ions, but become fully functional on the addition of the correct concentration of calcium salts.
Voltage gated sodium channels[edit]
Thevoltage gated sodium ion channelsin the cell membranes of nerves and muscle are particularly sensitive to the calcium ion concentration in the plasma.[6]Relatively small decreases in the plasma ionized calcium levels (hypocalcemia) cause these channels to leak sodium into the nerve cells or axons, making them hyper-excitable (positive bathmotropic effect), thus causing spontaneous muscle spasms (tetany) andparaesthesia(the sensation of "pins and needles" ) of the extremities and round the mouth.[7]When the plasma ionized calcium rises above normal (hypercalcemia) more calcium is bound to these sodium channels having a negative bathmotropic effect on them, causing lethargy, muscle weakness, anorexia, constipation and labile emotions.[7]
Intracellular signalling[edit]
Because the intracellular calcium ion concentration is extremely low (see above) the entry of minute quantities of calcium ions from the endoplasmic reticulum or from the extracellular fluids, cause rapid, very marked, and readily reversible changes in the relative concentration of these ions in thecytosol.This can therefore serve as a very effective intracellular signal (or "second messenger") in a variety of circumstances, includingmuscle contraction,the release of hormones (e.g.insulinfrom the beta cells in thepancreatic islets) or neurotransmitters (e.g.acetylcholinefrom pre-synaptic terminals of nerves) and other functions.
Bone[edit]
Calcium acts structurally assupporting materialin bones ascalcium hydroxyapatite(Ca10(PO4)6(OH)2).
Muscle[edit]
Inskeletalandheart muscle,calcium ions, released from thesarcoplasmic reticulum(theendoplasmic reticulumofstriated muscles), bind to thetroponin Cprotein present on theactin-containing thin filaments of themyofibrils.The troponin's3D structure changesas a result, causing thetropomyosinto which it is attached to be rolled away from themyosin-binding sites on theactin moleculesthat form the back-bone of the thin filaments.Myosincan then bind to the exposed myosin-binding sites on the thin filament, to undergo a repeating series of conformational changes called thecross-bridge cycle,for whichATPprovides the energy. During the cycle, each myosin protein ‘paddles’ along the thin actin filament, repeatedly binding to myosin-binding sites along the actin filament, ratcheting and letting go. In effect, the thick filament moves or slides along the thin filament, resulting inmuscle contraction.This process is known as thesliding filament modelof muscle contraction.[8][9][10][11][12]
Sources[edit]
Not all the calcium in the diet can be readily absorbed from the gut. The calcium that is most readily absorbed is found in dairy products (72%), vegetables (7%), grains (5%), legumes (4%), fruit (3%), protein (3%). The calcium contained in vegetable matter is often complexed withphytates,[13]oxalates,[14]citrateand other organic acids, such as the long-chained fatty acids (e.g.palmitic acid), with which calcium binds to form insoluble calcium soaps.[15]
Bone storage[edit]
Calcium flow to and from thebonemay be positive, negative, or neutral. When it is neutral, about 5–10 mmol is turned over a day. Bone serves as an important storage point for calcium, as it contains 99% of the total body calcium. Calcium release from bone is regulated byparathyroid hormonein conjunction withcalcitriolmanufactured in the kidney under the influence of PTH.Calcitonin(a hormone secreted by the thyroid gland when plasma ionized calcium levels are high or rising; not to be confused with "calcitriol" which is manufactured in the kidney) stimulates incorporation of calcium into bone.
Intestinal absorption[edit]
The normal adult diet contains about 25mmolof calcium per day. Only about 5 mmol of this is absorbed into the body per day (see below).[16]
Calcium is absorbed across the intestinal epithelial cell'sbrush bordermembrane. The TRPV6 channel was proposed to be the major player in intestinal Ca2+uptake.[17]However,Trpv6KO mice did not display significant reduction of serum calcium levels and showed only slightly reduced[17]or even unchanged intestinal Ca2+absorption,[18][19]indicating that other absorption pathways must exist. Recently,TRPM7was linked to intestinal calcium uptake. The authors could show that intestinal deletion ofTRPM7results in strongly reduced calcium levels in serum and bones,[20]and intensively increased levels ofcalcitriolandPTH,indicating thatTRPM7is essential for the intestinal bulk uptake of calcium. After the cellular uptake, calcium is immediately bound tocalbindin,avitamin D-dependent calcium-binding protein.Calbindin transfers the calcium directly into the epithelial cell'sendoplasmic reticulum,through which the calcium is transferred to thebasal membraneon the opposite side of the cell, without entering itscytosolor intracellular fluid. From there calcium pumps (PMCA1)actively transportcalcium into the body.[21]Active transport of calcium occurs primarily in theduodenumportion of the intestine when calcium intake is low; and through passiveparacellular transportin thejejunumandileumparts when calcium intake is high, independently of Vitamin D level.[22]
The active absorption of calcium from the gut is regulated by thecalcitriol(or 1,25 dihydroxycholecalciferol, or 1,25 dihydroxyvitamin D3) concentration in the blood. Calcitriol is a cholesterol derivative. Under the influence of ultraviolet light on the skin, cholesterol is converted to previtamin D3which spontaneously isomerizes to vitamin D3(or cholecalciferol). It is then converted from cholecalciferol to calcifediol in the liver.[23]Under the influence ofparathyroid hormone,thekidneysconvert calcifediol into the active hormone calcitriol, which acts on the epithelial cells (enterocytes) lining the small intestine to increase the rate of absorption of calcium from the intestinal contents. In short the cycle is following:
Low PTH levels in the blood (which occur under physiological conditions when the plasma ionized calcium levels are high) inhibit the conversion of cholecalciferol into calcitriol, which in turn inhibits calcium absorption from the gut. The opposite happens when the plasma ionized calcium levels are low: parathyroid hormone is secreted into the blood and the kidneys convert more calcifediol into the active calcitriol, increasing calcium absorption from the gut.[24]
Reabsorption[edit]
Intestine[edit]
Since about 15 mmol of calcium is excreted into the intestine via the bile per day,[4]the total amount of calcium that reaches the duodenum and jejunum each day is about 40 mmol (25 mmol from the diet plus 15 mmol from the bile), of which, on average, 20 mmol is absorbed (back) into the blood. The net result is that about 5 mmol more calcium is absorbed from the gut than is excreted into it via the bile. If there is no active bone building (as in childhood), or increased need for calcium during pregnancy and lactation, the 5 mmol calcium that is absorbed from the gut makes up for urinary losses that are only partially regulated.[16]
Kidneys[edit]
Thekidneysfilter 250 mmol of calcium ions a day in pro-urine (orglomerular filtrate), and resorbs 245 mmol, leading to a net average loss in the urine of about 5 mmol/d. The quantity of calcium ions excreted in the urine per day is partially under the influence of the plasmaparathyroid hormone(PTH) level - high levels of PTH decreasing the rate of calcium ion excretion, and low levels increasing it.[note 1]However, parathyroid hormone has a greater effect on the quantity ofphosphate ions(HPO42−) excreted in the urine.[25]Phosphates form insoluble salts in combination with calcium ions. High concentrations of HPO42−in the plasma, therefore, lower the ionized calcium level in the extra-cellular fluids. Thus, the excretion of more phosphate than calcium ions in the urine raises the plasma ionized calcium level, even though the total calcium concentration might be lowered.
The kidney influences the plasma ionized calcium concentration in yet another manner. It processesvitamin D3intocalcitriol,the active form that is most effective in promoting the intestinal absorption of calcium. This conversion of vitamin D3into calcitriol, is also promoted by high plasma parathyroid hormone levels.[24][26]
Excretion[edit]
Intestine[edit]
Most excretion of excess calcium is via the bile and feces, because the plasma calcitriol levels (which ultimately depend on the plasma calcium levels) regulate how much of the biliary calcium is reabsorbed from the intestinal contents.
Kidneys[edit]
Urinary excretion of calcium is normally about 5 mmol (200 mg) /day. This is less in comparison to what is excreted via the feces (15 mmol/day).
Regulation[edit]
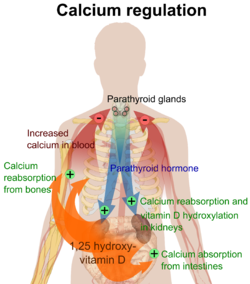
The plasma ionized calcium concentration is regulated within narrow limits (1.3–1.5 mmol/L). This is achieved by both theparafollicular cellsof the thyroid gland, and theparathyroid glandsconstantly sensing (i.e. measuring) the concentration of calcium ions in the blood flowing through them.
High plasma level[edit]
When the concentration of calcium rises, the parafollicular cells of the thyroid gland increase their secretion ofcalcitonin,a polypeptide hormone, into the blood. At the same time, the parathyroid glands reduce the secretion of parathyroid hormone (PTH), also a polypeptide hormone, into the blood. The resulting high levels of calcitonin in the blood stimulateosteoblastsin bone to remove calcium from blood plasma and deposit it as bone.
The reduced levels of PTH inhibit removal of calcium from the skeleton. The low levels of PTH have several other effects: there is increased loss of calcium in the urine, but more importantly, the loss of phosphate ions through urine is inhibited. Phosphate ions will therefore be retained in the plasma where they form insoluble salts with calcium ions, thereby removing them from the ionized calcium pool in the blood. The low levels of PTH also inhibit the formation ofcalcitriol(not to be confused withcalcitonin) from cholecalciferol (vitamin D3) by the kidneys.
The reduction in the blood calcitriol concentration acts (comparatively slowly) on the epithelial cells (enterocytes) of the duodenum, inhibiting their ability to absorb calcium from the intestinal contents.[2][5][28][29]The low calcitriol levels also act on bone causing theosteoclaststo release fewer calcium ions into the blood plasma.[25]

Low plasma level[edit]
When the plasma ionized calcium level is low or falls the opposite happens. Calcitonin secretion is inhibited and PTH secretion is stimulated, resulting in calcium being removed from bone to rapidly correct the plasma calcium level. The high plasma PTH levels inhibit calcium loss via the urine while stimulating the excretion of phosphate ions via that route. They also stimulate the kidneys to manufacture calcitriol (a steroid hormone), which enhances the ability of the cells lining the gut to absorb calcium from the intestinal contents into the blood, by stimulating the production ofcalbindinin these cells. The PTH stimulated production of calcitriol also causes calcium to be released from bone into the blood, by the release ofRANKL(acytokine,orlocal hormone) from theosteoblastswhich increases the bone resorptive activity by the osteoclasts. These are, however, relatively slow processes[2][5][25][28][29]
Thus fast short term regulation of the plasma ionized calcium level primarily involves rapid movements of calcium into or out of the skeleton. Long term regulation is achieved by regulating the amount of calcium absorbed from the gut or lost via the feces.[2][5][28][29]
Disorders[edit]
Hypocalcemia(low blood calcium) andhypercalcemia(high blood calcium) are both serious medical disorders.Osteoporosis,osteomalaciaandricketsare bone disorders linked to calcium metabolism disorders and effects ofvitamin D.Renal osteodystrophyis a consequence ofchronic kidney failurerelated to the calcium metabolism.
A diet adequately rich in calcium may reduce calcium loss from bone with advancing (post-menopausal) age.[30]A low dietary calcium intake may be a risk factor in the development ofosteoporosisin later life; and a diet with sustained adequate amounts of calcium may reduce the risk of osteoporosis.
Research[edit]
The role that calcium might have in reducing the rates of colorectal cancer has been the subject of many studies. However, given its modest efficacy, there is no current medical recommendation to use calcium for cancer reduction.
See also[edit]
Footnotes[edit]
- ^The main determinant of the amount of calcium excreted into the urine per day is the plasma ionized calcium concentration. The plasma parathyroid hormone (PTH) concentration only increases or decreases the amount of calcium excreted at anygiven plasma ionized calcium concentration.Thus, in primaryhyperparathyroidismthe quantity of calcium excreted in the urine per day isincreaseddespite the high levels of PTH in the blood. This is because hyperparathyroidism results inhypercalcemia,which increases the urinary calcium concentration (hypercalcuria) despite the modestly increased rate of calcium re-absorption from the renal tubules caused by PTH's effect on those tubules.Kidney stonesare therefore often a first indication of hyperparathyroidism, especially since the hypercalcuria is accompanied by an increase in urinary phosphate excretion (a direct result of the high plasma PTH levels). Together the calcium and phosphate tend to precipitate out as water-insoluble salts, which readily form solid “stones”.
References[edit]
- ^Marieb E (2000),Essentials of human anatomy and physiology,San Francisco: Benjamin Cummings,ISBN978-0805349405
- ^abcdBrini M, Ottolini D, Calì T, Carafoli E (2013). "Chapter 4. Calcium in Health and Disease". In Sigel A, Helmut RK (eds.).Interrelations between Essential Metal Ions and Human Diseases.Metal Ions in Life Sciences. Vol. 13. Springer. pp. 81–137.doi:10.1007/978-94-007-7500-8_4.ISBN978-94-007-7499-5.PMID24470090.
- ^abPeacock M (2010-01-01)."Calcium Metabolism in Health and Disease".Clinical Journal of the American Society of Nephrology.5(Supplement 1): S23–S30.doi:10.2215/CJN.05910809.ISSN1555-9041.PMID20089499.
- ^abcdDiem K, Lenter C.Scientific Tables.Vol. 565 (Seventh ed.). Basel: Ciba-Geigy Limited. pp. 653–654.ISBN978-3-9801244-0-9.
- ^abcdMarshall WJ (1995).Clinical Chemistry(3rd ed.). London: Mosby.ISBN978-0-7234-2190-0.
- ^Armstrong CM, Cota G (Mar 1999)."Calcium block of Na+ channels and its effect on closing rate".Proceedings of the National Academy of Sciences of the United States of America.96(7): 4154–7.Bibcode:1999PNAS...96.4154A.doi:10.1073/pnas.96.7.4154.PMC22436.PMID10097179.
- ^abHarrison TR.Principles of Internal Medicine(third ed.). New York: McGraw-Hill Book Company. pp. 170, 571–579.
- ^Silverthorn DU (2016). "Muscles".Human Physiology: An Integrated Approach(7th ed.). San Francisco, CA: Pearson. pp. 377–416.ISBN978-0-321-98122-6.
- ^Cooke R (June 2004)."The sliding filament model: 1972-2004".The Journal of General Physiology.123(6): 643–56.doi:10.1085/jgp.200409089.PMC2234572.PMID15173218.
- ^Geeves MA (January 2002). "Stretching the lever-arm theory".Nature.415(6868): 129–31.Bibcode:2002Natur.415..129G.doi:10.1038/415129a.PMID11805818.S2CID30618615.
- ^Spudich JA (November 1989)."In pursuit of myosin function".Cell Regulation.1(1): 1–11.doi:10.1091/mbc.1.1.1.PMC361420.PMID2519609.
- ^Yanagida T, Arata T, Oosawa F (1985). "Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle".Nature.316(6026): 366–9.Bibcode:1985Natur.316..366Y.doi:10.1038/316366a0.PMID4022127.S2CID4352361.
- ^Graf E (1983). "Calcium binding to phytic acid".Journal of Agricultural and Food Chemistry.31(4): 851–855.doi:10.1021/jf00118a045.
- ^Watts PS (2009). "Effects of oxalic acid ingestion by sheep. II. Large doses to sheep on different diets".The Journal of Agricultural Science.52(2): 250–255.doi:10.1017/S0021859600036765.S2CID86290753.
- ^López-López A, Castellote-Bargalló AI, Campoy-Folgoso C, Rivero-Urgël M, Tormo-Carnicé R, Infante-Pina D, López-Sabater MC (Nov 2001). "The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces".Early Human Development.65 Suppl: S83–94.doi:10.1016/S0378-3782(01)00210-9.PMID11755039.
- ^abBarrett KE, Barman SM, Boitano S, Brooks H, "Chapter 23. Hormonal Control of Calcium & Phosphate Metabolism & the Physiology of Bone" (Chapter). Barrett KE, Barman SM, Boitano S, Brooks H: Ganong's Review of Medical Physiology, 23e:http:// accessmedicine /content.aspx?aID=5244785Archived2011-07-07 at theWayback Machine.
- ^abBianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA (February 2007)."Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene".Journal of Bone and Mineral Research.22(2): 274–85.doi:10.1359/jbmr.061110.PMC4548943.PMID17129178.
- ^Sylvia Benn, Bryan S. Ajibade, Dare Porta, Angela Dhawan, Puneet Hediger, Matthias Peng, Ji-Bin Jiang, Yi Oh, Goo Taeg Jeung, Eui-Bae Lieben, Liesbet Bouillon, Roger Carmeliet, Geert Christakos.Active Intestinal Calcium Transport in the Absence of Transient Receptor Potential Vanilloid Type 6 and Calbindin-D9k.The Endocrine Society.OCLC680131487.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF (December 2008)."TRPV6 is not required for 1 Alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo".Proceedings of the National Academy of Sciences of the United States of America.105(50): 19655–9.Bibcode:2008PNAS..10519655K.doi:10.1073/pnas.0810761105.PMC2605002.PMID19073913.
- ^Mittermeier L, Demirkhanyan L, Stadlbauer B, Breit A, Recordati C, Hilgendorff A, Matsushita M, Braun A, Simmons DG, Zakharian E, Gudermann T, Chubanov V (February 2019)."TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival"(PDF).Proceedings of the National Academy of Sciences of the United States of America.116(10): 4706–4715.Bibcode:2019PNAS..116.4706M.doi:10.1073/pnas.1810633116.PMC6410795.PMID30770447.
- ^Balesaria S, Sangha S, Walters JR (December 2009)."Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption".American Journal of Physiology. Gastrointestinal and Liver Physiology.297(6): G1193-7.doi:10.1152/ajpgi.00237.2009.PMC2850091.PMID19779013.
- ^"Absorption of Minerals and Metals".vivo.colostate.edu.Retrieved19 April2018.
- ^Brandi M (2010)."Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes".Clinical Cases in Mineral and Bone Metabolism.7(3): 243–250.ISSN1724-8914.PMC3213838.PMID22460535.
- ^abStryer L.Biochemistry(Fourth Edition). Chapter 27 "Vitamin D is derived from cholesterol by the ring-splitting action of light". New York, W.H. Freeman and Company.
- ^abcBlaine J, Chonchol M, Levi M (2015)."Renal control of calcium, phosphate, and magnesium homeostasis".Clinical Journal of the American Society of Nephrology.10(7): 1257–72.doi:10.2215/CJN.09750913.PMC4491294.PMID25287933.
- ^Tortora GJ, Anagnostakos NP.Principles of Anatomy and Physiology(Fifth Edition) p. 696. New York, Harper & Row Publishers.
- ^Boron, Walter F., Boulpaep, Emile L (2003). "The Parathyroid Glands and Vitamin D".Medical Physiology: A Cellular And Molecular Approach.Elsevier/Saunders. p. 1094.ISBN978-1-4160-2328-9.
- ^abcWalter F. (2003). "The Parathyroid Glands and Vitamin D in".Medical Physiology: A Cellular And Molecular Approach.Elsevier/Saunders. p. 1094.ISBN978-1-4160-2328-9.
- ^abcGuyton A (1976). ‘’Medical Physiology’’. p.1062; New York, Saunders and Co.
- ^Heaney RP (Apr 2000)."Calcium, dairy products and osteoporosis".Journal of the American College of Nutrition.19(2 Suppl): 83S–99S.doi:10.1080/07315724.2000.10718088.PMID10759135.S2CID18794160.Archived fromthe originalon 2012-08-03.
External links[edit]
- Calciumat Lab Tests Online
- Nosek TM."Section 5/5ch6/5ch6line".Essentials of Human Physiology.[dead link]

