Carbon-14
 | |
| General | |
|---|---|
| Symbol | 14C |
| Names | carbon-14, 14C, C-14, radiocarbon |
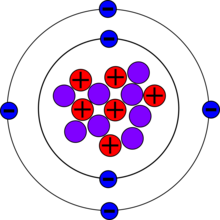
| Protons(Z) | 6 |
| Neutrons(N) | 8 |
| Nuclide data | |
| Natural abundance | 1 part per trillion = |
| Half-life(t1/2) | 5700±30 years[1] |
| Isotope mass | 14.0032420[2]Da |
| Spin | 0+ |
| Decay modes | |
| Decay mode | Decay energy(MeV) |
| Beta | 0.156476[2] |
| Isotopes of carbon Complete table of nuclides | |
Carbon-14,C-14,14
Corradiocarbon,is aradioactive isotopeofcarbonwith anatomic nucleuscontaining 6protonsand 8neutrons.Its presence in organic materials is the basis of theradiocarbon datingmethod pioneered byWillard Libbyand colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, byMartin KamenandSam Rubenat theUniversity of California Radiation LaboratoryinBerkeley, California.Its existence had been suggested byFranz Kuriein 1934.[3]
There are three naturally occurringisotopesof carbon on Earth:carbon-12(12
C), which makes up 99% of all carbon on Earth;carbon-13(13
C), which makes up 1%; and carbon-14 (14
C), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has ahalf-lifeof5700±30years.[4]Carbon-14 has a maximum specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g.[5]Carbon-14 decays intonitrogen-14(14
N) throughbeta decay.[6]A gram of carbon containing 1 atom of carbon-14 per 1012atoms will emit ~0.2[7]beta particles per second. The primary natural source of carbon-14 on Earth iscosmic rayaction on nitrogen in the atmosphere, and it is therefore acosmogenic nuclide.However, open-airnuclear testingbetween 1955 and 1980 contributed to this pool.
The different isotopes of carbon do not differ appreciably in their chemical properties. This resemblance is used in chemical and biological research, in a technique calledcarbon labeling:carbon-14 atoms can be used to replace nonradioactive carbon, in order to trace chemical and biochemical reactions involving carbon atoms from any given organic compound.
Radioactive decay and detection[edit]
Carbon-14 goes through radioactivebeta decay:
- 14
6C→14
7N+e−+
ν
e+ 156.5 keV
By emitting anelectronand anelectron antineutrino,one of the neutrons in the carbon-14 atom decays to a proton and the carbon-14 (half-lifeof 5,700 ± 30 years[1]) decays into the stable (non-radioactive) isotopenitrogen-14.
As usual with beta decay, almost all the decay energy is carried away by the beta particle and the neutrino. The emitted beta particles have a maximum energy of about 156 keV, while their weighted mean energy is 49 keV.[8]These are relatively low energies; the maximum distance traveled is estimated to be 22 cm in air and 0.27 mm in body tissue. The fraction of the radiation transmitted through thedead skin layeris estimated to be 0.11. Small amounts of carbon-14 are not easily detected by typicalGeiger–Müller (G-M) detectors;it is estimated that G-M detectors will not normally detect contamination of less than about 100,000 disintegrations per minute (0.05 μCi).Liquid scintillation countingis the preferred method[9]although more recently, accelerator mass spectrometry has become the method of choice; it counts all the carbon-14 atoms in the sample and not just the few that happen to decay during the measurements; it can therefore be used with much smaller samples (as small as individual plant seeds), and gives results much more quickly. The G-M counting efficiency is estimated to be 3%. The half-distance layer in water is 0.05 mm.[10]
Radiocarbon dating[edit]
Radiocarbon dating is aradiometric datingmethod that uses (14
C) to determine the age ofcarbonaceousmaterials up to about 60,000 years old. The technique was developed byWillard Libbyand his colleagues in 1949[11]during his tenure as a professor at theUniversity of Chicago.Libby estimated that the radioactivity of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram of pure carbon, and this is still used as the activity of themodern radiocarbon standard.[12][13]In 1960, Libby was awarded theNobel Prize in chemistryfor this work.
One of the frequent uses of the technique is to date organic remains from archaeological sites. Plantsfixatmospheric carbon during photosynthesis, so the level of14
Cin plants and animals when they die approximately equals the level of14
Cin the atmosphere at that time. However, it decreases thereafter from radioactive decay, allowing the date of death or fixation to be estimated. The initial14
Clevel for the calculation can either be estimated, or else directly compared with known year-by-year data from tree-ring data (dendrochronology) up to 10,000 years ago (using overlapping data from live and dead trees in a given area), or else from cave deposits (speleothems), back to about 45,000 years before the present. A calculation or (more accurately) a direct comparison of carbon-14 levels in a sample, with tree ring or cave-deposit carbon-14 levels of a known age, then gives the wood or animal sample age-since-formation. Radiocarbon is also used to detect disturbance in natural ecosystems; for example, inpeatlandlandscapes, radiocarbon can indicate that carbon which was previously stored in organic soils is being released due to land clearance or climate change.[14][15]
Cosmogenic nuclides are also used asproxydata to characterize cosmic particle and solar activity of the distant past.[16][17]
Origin[edit]
Natural production in the atmosphere[edit]

2: Decay of carbon-14
3: The "equal" equation is for living organisms, and the unequal one is for dead organisms, in which the C-14 then decays (See 2).
Carbon-14 is produced in the uppertroposphereand thestratospherebythermal neutronsabsorbed bynitrogenatoms. Whencosmic raysenter the atmosphere, they undergo various transformations, including the production ofneutrons.The resulting neutrons (n) participate in the followingn-preaction (p isproton):
- 14
7N+ n →14
6C+ p
The highest rate of carbon-14 production takes place at altitudes of 9 to 15 kilometres (30,000 to 49,000 ft) and at highgeomagnetic latitudes.
The rate of14
Cproduction can be modelled, yielding values of 16,400[18]or 18,800[19]atoms of14
Cper second per square meter of the Earth's surface, which agrees with the globalcarbon budgetthat can be used to backtrack,[20]but attempts to measure the production time directlyin situwere not very successful. Production rates vary because of changes to the cosmic ray flux caused by the heliospheric modulation (solar wind and solar magnetic field), and, of great significance, due to variations in theEarth's magnetic field.Changes in thecarbon cyclehowever can make such effects difficult to isolate and quantify.
[20][21]
Occasional spikes may occur; for example, there is evidence foran unusually high production rate in AD 774–775,[22]caused by an extreme
solar energetic particle event, the strongest such event to have occurred within the last ten millennia.[23][24]Another "extraordinarily large"14
Cincrease (2%) has been associated with a 5480 BC event, which is unlikely to be a solar energetic particle event.[25]
Carbon-14 may also be produced by lightning[26][27]but in amounts negligible, globally, compared to cosmic ray production. Local effects of cloud-ground discharge through sample residues are unclear, but possibly significant.
Other carbon-14 sources[edit]
Carbon-14 can also be produced by other neutron reactions, including in particular13
C(n,γ)14
Cand17
O(n,α)14
Cwiththermal neutrons,and15
N(n,d)14
Cand16
O(n,3
He)14
Cwithfast neutrons.[28]The most notable routes for14
Cproduction by thermal neutron irradiation of targets (e.g., in a nuclear reactor) are summarized in the table.
Carbon-14 may also beradiogenic(cluster decayof223
Ra,224
Ra,226
Ra). However, this origin is extremely rare.
| Parent isotope | Natural abundance, % | Cross section for thermal neutron capture,b | Reaction |
|---|---|---|---|
| 14 N |
99.634 | 1.81 | 14 N(n,p)14 C |
| 13 C |
1.103 | 0.0009 | 13 C(n,γ)14 C |
| 17 O |
0.0383 | 0.235 | 17 O(n,α)14 C |
Formation during nuclear tests[edit]

C,New Zealand[30]andAustria.[31]The New Zealand curve is representative for the Southern Hemisphere, the Austrian curve is representative for the Northern Hemisphere. Atmospheric nuclear weapon tests almost doubled the concentration of14
Cin the Northern Hemisphere.[32]The annotated PTBT label is representative of thePartial Nuclear Test Ban Treaty.
The above-groundnuclear teststhat occurred in several countries between 1955 and 1980(see nuclear test list)dramatically increased the amount of carbon-14 in the atmosphere and subsequently in the biosphere; after the tests ended, the atmospheric concentration of the isotope began to decrease, as radioactiveCO2was fixed into plant and animal tissue, and dissolved in the oceans.
One side-effect of the change in atmospheric carbon-14 is that this has enabled some options (e.g.,bomb-pulse dating[33]) for determining the birth year of an individual, in particular, the amount of carbon-14 intooth enamel,[34][35]or the carbon-14 concentration in the lens of the eye.[36]
In 2019,Scientific Americanreported that carbon-14 from nuclear bomb testing has been found in the bodies of aquatic animals found in one of the most inaccessible regions of the earth, theMariana Trenchin the Pacific Ocean.[37]
The concentration of carbon-14 in atmospheric CO2,reported as the ratio of carbon-14 to carbon-12 with respect to a standard, has now (approximately since the year 2022) declined to levels similar to those prior to the above-ground nuclear tests of the 1950s and 1960s.[38][39]Although the extra carbon-14 atoms generated during those nuclear tests have not disappeared from the atmosphere, oceans and biosphere,[40]they are diluted because of theSuess effect.
Emissions from nuclear power plants[edit]
Carbon-14 is produced in coolant atboiling water reactors(BWRs) andpressurized water reactors(PWRs). It is typically released to the atmosphere in the form ofcarbon dioxideat BWRs, andmethaneat PWRs.[41]Best practice for nuclear power plant operator management of carbon-14 includes releasing it at night, when plants are notphotosynthesizing.[42]Carbon-14 is also generated inside nuclear fuels (some due to transmutation of oxygen in theuranium oxide,but most significantly from transmutation of nitrogen-14 impurities), and if the spent fuel is sent tonuclear reprocessingthen the carbon-14 is released, for example asCO2duringPUREX.[43][44]
Occurrence[edit]
Dispersion in the environment[edit]
After production in the upper atmosphere, the carbon-14 atoms react rapidly to form mostly (about 93%)14
CO(carbon monoxide), which subsequently oxidizes at a slower rate to form14
CO
2,radioactivecarbon dioxide.The gas mixes rapidly and becomes evenly distributed throughout the atmosphere (the mi xing timescale in the order of weeks). Carbon dioxide also dissolves in water and thus permeates theoceans,but at a slower rate.[21]The atmospheric half-life for removal of14
CO
2has been estimated to be roughly 12 to 16 years in the northern hemisphere. The transfer between the ocean shallow layer and the large reservoir ofbicarbonatesin the ocean depths occurs at a limited rate.[29]
In 2009 the activity of14
Cwas 238 Bq per kg carbon of fresh terrestrial biomatter, close to the values before atmospheric nuclear testing (226 Bq/kg C; 1950).[45]
Total inventory[edit]
The inventory of carbon-14 in Earth's biosphere is about 300megacuries(11EBq), of which most is in the oceans.[46] The following inventory of carbon-14 has been given:[47]
- Global inventory: ~8500 PBq (about 50t)
- Atmosphere: 140 PBq (840 kg)
- Terrestrial materials: the balance
- From nuclear testing (until 1990): 220 PBq (1.3 t)
In fossil fuels[edit]
Many human-made chemicals are derived fromfossil fuels(such aspetroleumorcoal) in which14
Cis greatly depleted because the age of fossils far exceeds the half-life of14
C.The relative absence of14
CO
2is therefore used to determine the relative contribution (ormi xing ratio) of fossil fuel oxidation to the totalcarbon dioxidein a given region of the Earth'satmosphere.[48]
Dating a specific sample of fossilized carbonaceous material is more complicated. Such deposits often contain trace amounts of carbon-14. These amounts can vary significantly between samples, ranging up to 1% of the ratio found in living organisms, a concentration comparable to an apparent age of 40,000 years.[49]This may indicate possible contamination by small amounts of bacteria, underground sources of radiation causing the14
N(n,p)14
Creaction, directuraniumdecay (although reported measured ratios of14
C/U in uranium-bearing ores[50]would imply roughly 1 uranium atom for every two carbon atoms in order to cause the14
C/12
Cratio, measured to be on the order of 10−15), or other unknown secondary sources of carbon-14 production. The presence of carbon-14 in theisotopic signatureof a sample of carbonaceous material possibly indicates its contamination by biogenic sources or the decay of radioactive material in surrounding geologic strata. In connection with building theBorexinosolar neutrino observatory, petroleum feedstock (for synthesizing the primary scintillant) was obtained with low14
Ccontent. In the Borexino Counting Test Facility, a14
C/12
Cratio of 1.94×10−18was determined;[51]probable reactions responsible for varied levels of14
Cin differentpetroleum reservoirs,and the lower14
Clevels in methane, have been discussed by Bonvicini et al.[52]
In the human body[edit]
Since many sources of human food are ultimately derived from terrestrial plants, the relative concentration of carbon-14 in human bodies is nearly identical to the relative concentration in the atmosphere. The rates of disintegration ofpotassium-40and carbon-14 in the normal adult body are comparable (a few thousand disintegrated nuclei per second).[53]The beta decays from external (environmental) radiocarbon contribute approximately 0.01mSv/year (1 mrem/year) to each person'sdoseofionizing radiation.[54]This is small compared to the doses from potassium-40 (0.39 mSv/year) andradon(variable).
Carbon-14 can be used as aradioactive tracerin medicine. In the initial variant of theurea breath test,a diagnostic test forHelicobacter pylori,urea labeled with approximately 37kBq(1.0μCi) carbon-14 is fed to a patient (i.e., 37,000 decays per second). In the event of aH. pyloriinfection, the bacterialureaseenzyme breaks down theureaintoammoniaand radioactively-labeledcarbon dioxide,which can be detected by low-level counting of the patient's breath.[55]
See also[edit]
References[edit]
- ^abKondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021)."The NUBASE2020 evaluation of nuclear properties"(PDF).Chinese Physics C.45(3): 030001.doi:10.1088/1674-1137/abddae.
- ^abWaptstra AH, Audi G, Thibault C."AME atomic mass evaluation 2003".IAEA.org.Archivedfrom the original on 5 May 2023.
- ^Kamen MD (May 1963). "Early History of Carbon-14: Discovery of this supremely important tracer was expected in the physical sense but not in the chemical sense".Science.140(3567): 584–590.Bibcode:1963Sci...140..584K.doi:10.1126/science.140.3567.584.PMID17737092.
- ^Kondev, F.G.; Wang, M.; Huang, W.J.; Naimi, S.; Audi, G. (2021-03-01)."The NUBASE2020 evaluation of nuclear physics properties *".Chinese Physics C.45(3): 030001.doi:10.1088/1674-1137/abddae.ISSN1674-1137.
- ^Babin V, Taran F, Audisio D (June 2022)."Late-Stage Carbon-14 Labeling and Isotope Exchange: Emerging Opportunities and Future Challenges".JACS Au.2(6): 1234–1251.doi:10.1021/jacsau.2c00030.PMC9241029.PMID35783167.
- ^"What is carbon dating?".National Ocean Sciences Accelerator Mass Spectrometry Facility. Archived fromthe originalon July 5, 2007.Retrieved2007-06-11.
- ^(1 per 1012) × (1 gram / (12 grams per mole)) × (Avogadro constant) / ((5,730 years) × (31,557,600 seconds perJulian year) /ln(2))
- ^Nicols AL."14C Comments on evaluation of decay data"(PDF).nucleide.org.LNHB.Archived(PDF)from the original on 2011-08-15.Retrieved30 October2021.
- ^"Appendix B: The Characteristics of Common Radioisotopes".Radiation Safety Manual for Laboratory Users.Princeton University. Archived fromthe originalon 2013-10-02.
- ^"Carbon-14".Material Safety Data Sheet.University of Michigan. Archived fromthe originalon 2013-03-12.
- ^Arnold JR, Libby WF (December 1949). "Age determinations by radiocarbon content; checks with samples of known age".Science.110(2869): 678–680.Bibcode:1949Sci...110..678A.doi:10.1126/science.110.2869.678.PMID15407879.
- ^"Carbon 14:age calculation".C14dating.Archivedfrom the original on 2007-06-10.Retrieved2007-06-11.
- ^"Class notes for Isotope Hydrology EESC W 4886: Radiocarbon14C ".Martin Stute's homepage at Columbia.Archivedfrom the original on 2006-09-24.Retrieved2007-06-11.
- ^Moore S, Evans CD, Page SE, Garnett MH, Jones TG, Freeman C, et al. (January 2013)."Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes"(PDF).Nature.493(7434): 660–663.Bibcode:2013Natur.493..660M.doi:10.1038/nature11818.PMID23364745.S2CID205232299.
- ^Dean JF, Garnett MH, Spyrakos E, Billett MF (2019)."The Potential Hidden Age of Dissolved Organic Carbon Exported by Peatland Streams".Journal of Geophysical Research: Biogeosciences.124(2): 328–341.Bibcode:2019JGRG..124..328D.doi:10.1029/2018JG004650.hdl:1893/28684.ISSN2169-8953.
- ^Reimer PJ, Austin WE, Bard E, Bayliss A, Blackwell PG, Ramsey CB, et al. (August 2020)."The INTCAL20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 CAL kBP)".Radiocarbon.62(4): 725–757.Bibcode:2020Radcb..62..725R.doi:10.1017/RDC.2020.41.hdl:11585/770531.
- ^Brehm N, Bayliss A, Christl M, Synal HA, Adolphi F, Beer J, et al. (2021)."Eleven-year solar cycles over the last millennium revealed by radiocarbon in tree rings".Nature Geoscience.14(1): 10–15.Bibcode:2021NatGe..14...10B.doi:10.1038/s41561-020-00674-0.S2CID230508539.
- ^Kovaltsov GA, Mishev A, Usoskin IG (2012). "A new model of cosmogenic production of radiocarbon 14C in the atmosphere".Earth and Planetary Science Letters.337–338: 114–20.arXiv:1206.6974.Bibcode:2012E&PSL.337..114K.doi:10.1016/j.epsl.2012.05.036.ISSN0012-821X.S2CID118602346.
- ^Poluianov SV, Kovaltsov GA, Mishev AL, Usoskin IG (2016). "Production of cosmogenic isotopes 7Be, 10Be, 14C, 22Na, and 36Cl in the atmosphere: Altitudinal profiles of yield functions".Journal of Geophysical Research: Atmospheres.121(13): 8125–36.arXiv:1606.05899.Bibcode:2016JGRD..121.8125P.doi:10.1002/2016JD025034.S2CID119301845.
- ^abHain MP, Sigman DM, Haug GH (2014)."Distinct roles of the Southern Ocean and North Atlantic in the deglacial atmospheric radiocarbon decline"(PDF).Earth and Planetary Science Letters.394:198–208.Bibcode:2014E&PSL.394..198H.doi:10.1016/j.epsl.2014.03.020.ISSN0012-821X.Archived(PDF)from the original on 2015-12-22.
- ^abRamsey, C. Bronk (2008). "Radiocarbon Dating: Revolutions in Understanding".Archaeometry.50(2): 249–75.doi:10.1111/j.1475-4754.2008.00394.x.
- ^Miyake F, Nagaya K, Masuda K, Nakamura T (June 2012)."A signature of cosmic-ray increase in AD 774-775 from tree rings in Japan"(PDF).Nature.486(7402): 240–242.Bibcode:2012Natur.486..240M.doi:10.1038/nature11123.PMID22699615.S2CID4368820.Archived fromthe original(PDF)on 2015-07-06.
- ^Usoskin IG, Kromer B, Ludlow F, Beer J, Friedrich M, Kovaltsov GA, et al. (2013). "The AD775 cosmic event revisited: the Sun is to blame".Astron. Astrophys.552:L3.arXiv:1302.6897.Bibcode:2013A&A...552L...3U.doi:10.1051/0004-6361/201321080.S2CID55137950.
- ^Mekhaldi F, Muscheler R, Adolphi F, Aldahan A, Beer J, McConnell JR, et al. (October 2015)."Multiradionuclide evidence for the solar origin of the cosmic-ray events of ᴀᴅ 774/5 and 993/4".Nature Communications.6:8611.Bibcode:2015NatCo...6.8611M.doi:10.1038/ncomms9611.PMC4639793.PMID26497389.
- ^Miyake F, Jull AJ, Panyushkina IP, Wacker L, Salzer M, Baisan CH, et al. (January 2017)."Large 14C excursion in 5480 BC indicates an abnormal sun in the mid-Holocene".Proceedings of the National Academy of Sciences of the United States of America.114(5): 881–884.Bibcode:2017PNAS..114..881M.doi:10.1073/pnas.1613144114.PMC5293056.PMID28100493.
- ^Libby LM, Lukens HR (1973). "Production of radiocarbon in tree rings by lightning bolts".Journal of Geophysical Research.78(26): 5902–5903.Bibcode:1973JGR....78.5902L.doi:10.1029/JB078i026p05902.
- ^Enoto T, Wada Y, Furuta Y, Nakazawa K, Yuasa T, Okuda K, et al. (November 2017). "Photonuclear reactions triggered by lightning discharge".Nature.551(7681): 481–484.arXiv:1711.08044.Bibcode:2017Natur.551..481E.doi:10.1038/nature24630.PMID29168803.S2CID4388159.
- ^Davis Jr W (January 1977).Carbon-14 production in nuclear reactors.U.S. Nuclear Regulatory Commission(Report). TN (USA): Oak Ridge National Lab.doi:10.2172/7114972.
- ^abYim MS, Caron F (2006). "Life cycle and management of carbon-14 from nuclear power generation".Progress in Nuclear Energy.48:2–36.doi:10.1016/j.pnucene.2005.04.002.
- ^Manning MR, Melhuish WH (1994)."Atmospheric δ14C record from Wellington ".Trends: A Compendium of Data on Global Change.Carbon Dioxide Information Analysis Center. Archived fromthe originalon 2014-02-01.Retrieved2007-06-11.
- ^Levin I, Kromer B, Schoch-Fischer H, Bruns M, Münnich M, Berdau D, Vogel JW, Münnich KO (1994)."δ14C record from Vermunt ".Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center.Archived fromthe originalon 2008-09-23.Retrieved2009-03-25.
- ^"Radiocarbon dating".University of Utrecht.Archivedfrom the original on 2007-12-09.Retrieved2008-02-19.
- ^Stenstrom K, Georgiadou E (August 2010)."Bomb-Pulse Dating of Human Material: Modeling the Influence of Diet".Radiocarbon.52(2): 800–07.Bibcode:2010Radcb..52..800G.doi:10.1017/S0033822200045811.Archivedfrom the original on 2014-10-20.
- ^"Radiation in Teeth Can Help Date, ID Bodies, Experts Say".National Geographic News.2005-09-22. Archived fromthe originalon 2007-04-25.
- ^Spalding KL, Buchholz BA, Bergman LE, Druid H, Frisén J (September 2005). "Forensics: age written in teeth by nuclear tests".Nature.437(7057): 333–334.Bibcode:2005Natur.437..333S.doi:10.1038/437333a.PMID16163340.S2CID4407447.
- ^Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J (January 2008). Gazit E (ed.)."Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life".PLOS ONE.3(1): e1529.Bibcode:2008PLoSO...3.1529L.doi:10.1371/journal.pone.0001529.PMC2211393.PMID18231610.
- ^Levy A (15 May 2019)."'Bomb Carbon' Has Been Found in Deep-Ocean Creatures ".Scientific American.
- ^Jones, Nicola (27 July 2022)."Carbon dating hampered by rising fossil-fuel emissions".Nature News.Retrieved5 November2023.
- ^Graven, H.; Keeling, R.; Xu, X. (19 July 2022)."Radiocarbon dating: going back in time".Nature.607(7919): 449.Bibcode:2022Natur.607R.449G.doi:10.1038/d41586-022-01954-y.PMID35854150.
- ^Caldeira, K.; Rau, G.H.; Duffy, PB (1998)."Predicted net efflux of radio- carbon from the ocean and increase in atmospheric radiocarbon content".Geophysical Research Letters.25(20): 3811–3814.Bibcode:1998GeoRL..25.3811C.doi:10.1029/1998GL900010.
- ^"EPRI | Product Abstract | Impact of Nuclear Power Plant Operations on Carbon-14 Generation, Chemical Forms, and Release".epri.Archived fromthe originalon 2016-08-18.Retrieved2016-07-07.
- ^"EPRI | Product Abstract | Carbon-14 Dose Calculation Methods at Nuclear Power Plants".epri.Archived fromthe originalon 2016-08-18.Retrieved2016-07-07.
- ^Otlet RL, Fulker MJ, Walker AJ (1992). "Environmental Impact of Atmospheric Carbon-14 Emissions Resulting from the Nuclear Energy Cycle.". In Taylor RE, Long A, Kra RS (eds.).Radiocarbon After Four Decades.New York, NY: Springer.
- ^"Carbon-14 and the environment".Institute for Radiological Protection and Nuclear Safety.
- ^"Carbon-14 and the environment".Institute for Radiological Protection and Nuclear Safety.Archivedfrom the original on 2015-04-18.
- ^"Human Health Fact Sheet – Carbon 14"(PDF).Argonne National Laboratory, EVS. August 2005. Archived fromthe original(PDF)on 2011-07-16.
- ^Choppin GR,Liljenzin JO,Rydberg J (2002).Radiochemistry and Nuclear Chemistry(3rd ed.). Butterworth-Heinemann.ISBN978-0-7506-7463-8.
- ^"The Basics: 14C and Fossil Fuels".NOAA ESRL GMD Education and Outreach.Archived fromthe originalon 25 September 2015.Retrieved9 Dec2015.
All other atmospheric carbon dioxide comes from young sources–namely land-use changes (for example, cutting down a forest in order to create a farm) and exchange with the ocean and terrestrial biosphere. This makes 14C an ideal tracer of carbon dioxide coming from the combustion of fossil fuels. Scientists can use 14C measurements to determine the age of carbon dioxide collected in air samples, and from this can calculate what proportion of the carbon dioxide in the sample comes from fossil fuels.
- ^Lowe D (1989)."Problems associated with the use of coal as a source of C14-free background material".Radiocarbon.31(2): 117–120.Bibcode:1989Radcb..31..117L.doi:10.1017/S0033822200044775.Archivedfrom the original on 2013-07-24.
- ^Jull AJ, Barker D, Donahue DJ (1985). "Carbon-14 Abundances in Uranium Ores and Possible Spontaneous Exotic Emission from U-Series Nuclides".Meteoritics.20:676.Bibcode:1985Metic..20..676J.
- ^Alimonti G, Angloher G, Arpesella C, Balata M, Bellini G, Benziger J, et al. (1998). "Measurement of the14C abundance in a low-background liquid scintillator ".Physics Letters B.422(1–4): 349–358.Bibcode:1998PhLB..422..349B.doi:10.1016/S0370-2693(97)01565-7.
- ^Bonvicini G, Harris N, Paolone V (2003). "The chemical history of14C in deep oilfields ".arXiv:hep-ex/0308025.
- ^Rowland RE."The Radioactivity of the Normal Adult Body".rerowland.Archived fromthe originalon 2011-02-05.
- ^Ionizing Radiation Exposure of the Population of the United States | NCRP Report No. 93.National Council on Radiation Protection and Measurements. 1987. Archived fromthe originalon 2007-07-11.)
- ^"Society of Nuclear Medicine Procedure Guideline for C-14 Urea Breath Test"(PDF).snm.org.2001-06-23. Archived fromthe original(PDF)on 2007-09-26.Retrieved2007-07-04.
Further reading[edit]
- Kamen MD (1985).Radiant Science, Dark Politics: A Memoir of the Nuclear Age.Berkeley: University of California Press.ISBN978-0-520-04929-1.
External links[edit]

