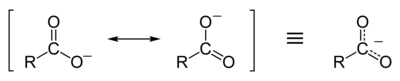
Carboxylate


Inorganic chemistry,acarboxylateis theconjugate baseof acarboxylic acid,RCOO−(orRCO−2). It is ananion,anionwithnegative charge.
Carboxylate saltsaresaltsthat have the general formulaM(RCOO)n,where M is a metal andnis 1, 2,....Carboxylate estershave the general formulaRCOOR′(also written asRCO2R′), where R and R′ are organic groups.
Synthesis
[edit]Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically havepKaof less than 5, meaning that they can be deprotonated by many bases, such assodium hydroxideorsodium bicarbonate.[1]: 271–2
Resonance stabilization of the carboxylate ion
[edit]Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into analkoxideion and a proton), because the carboxylate ion is stabilized byresonance.The negative charge that is left afterdeprotonationof the carboxyl group is delocalized between the twoelectronegativeoxygen atoms in a resonance structure. If the R group is an electron-withdrawing group (such as–CF3), the basicity of the carboxylate will be further weakened.[1]: 264–5
Thisdelocalizationof the electron means that both of the oxygen atoms are less strongly negatively charged: the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result ofresonance stabilizationof the negative charge. In contrast, analkoxideion, once formed, would have a strong negative charge localized on its lone oxygen atom, which would strongly attract any nearby protons (indeed, alkoxides are very strong bases). Because of resonance stabilization, carboxylic acids have much lowerpKavalues (and are therefore stronger acids) thanalcohols.For example, the pKavalue ofacetic acidis 4.8, whileethanolhas a pKaof 16. Hence acetic acid is a much stronger acid than ethanol. This in turn means that for equimolar solutions of a carboxylic acid or an alcohol in water, the carboxylic acid would have a much lowerpH.[1]: 263–7
Reactions
[edit]Alkyation
[edit]Carboxylic acid salts with a hydrogen atom in theAlphaposition next to the carboxylate group can be converted todianionswith strong bases likelithium diisopropylamide.These react withalkyl halidesto give derivatives:[1]: 474
- RCH2COO−+ Li+[−N(CH(CH3)2)2] → RCH−COO−
- RCH−COO−+ R'X → RR'CHCOO−+ X−
Nucleophilic substitution
[edit]Carboxylate ions are goodnucleophiles.They react withalkyl halidesto formesters.The following reaction shows the reaction mechanism.[1]: 398–9
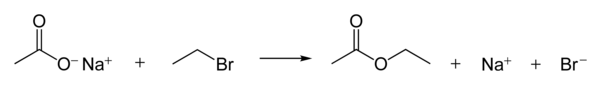
The nucleophilicity of carboxylate ions is much weaker than that ofhydroxideandalkoxideions, but stronger than that ofhalideanions (in apolar aprotic solvent,though there are other effects such assolubilityof the ion).
Reduction
[edit]Unlike the reduction of ester, the reduction of carboxylate is different, due to the lack of theleaving groupand the relatively electron-rich carbon atom (due to the negative charge on the oxygen atoms). With a small amount of acid, the reaction occurs withlithium aluminium hydrideby changing the LAH into theLewis acidAlH3in the process, converting the oxyanion to 4 Al–O bonds.[1]: 1212
Examples
[edit]This list is for cases where there is a separate article for the anion or its derivatives. All other organic acids should be found at their parent carboxylic acid.
- Formateion, HCOO−
- Acetateion, CH3COO−
- Methanetetracarboxylateion, C(COO−)4
- Oxalateion,(COO)2−
2
See also
[edit]References
[edit]- ^abcdefMarch, Jerry(1992),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(4th ed.), New York: Wiley,ISBN0-471-60180-2