Carotid body
| Carotid body | |
|---|---|
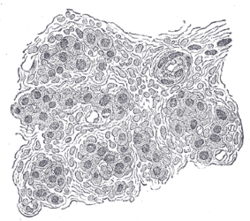 Section of part of human carotid body. Highly magnified. Numerous blood vessels are seen in section among the cells. | |
 Diagram showing the origins of the main branches of the carotid arteries. | |
| Details | |
| Nerve | Branch of glossopharyngeal nerve to carotid sinus |
| Identifiers | |
| Latin | glomus caroticum |
| MeSH | D002344 |
| TA98 | A12.2.04.007 |
| TA2 | 3886 |
| FMA | 50095 |
| Anatomical terminology | |
Thecarotid bodyis a small cluster ofperipheral chemoreceptorcells and supportingsustentacular cellssituated at the bifurcation of eachcommon carotid arteryin itstunica externa.[1][2]
The carotid body detects changes in the composition ofarterial bloodflowing through it, mainly thepartial pressure of arterial oxygen,but also ofcarbon dioxide.It is also sensitive to changes inblood pH,andtemperature.
Structure
[edit]The carotid body is situated on the posterior aspect of the bifurcation of the common carotid artery.[3]
The carotid body is made up of two types of cells, calledglomus cells:glomus type I cells areperipheral chemoreceptors,and glomus type II cells aresustentacularsupportive cells.
- Glomus type I cells are derived from theneural crest.[4]They release a variety ofneurotransmitters,includingacetylcholine,ATP,anddopaminethat triggerEPSPsin synapsed neurons leading to therespiratory center.They are innervated by axons of the glossopharyngeal nerve which collectively are called the carotid sinus nerve.
- Glomus type II cells resembleglial cells,express the glial markerS100and act as supporting cells.
Function
[edit]This sectionneeds morereliable medical referencesforverificationor relies too heavily onprimary sources.(October 2019) |  |
The carotid body functions as a sensor: it responds to a stimulus, primarily O2partial pressure, which is detected by the type I (glomus) cells, and triggers anaction potentialthrough theafferent fibersof theglossopharyngeal nerve,which relays the information to the central nervous system.
Stimulus
[edit]The carotid bodyperipheral chemoreceptorsare primarily sensitive to decreases in the partial pressure of oxygen (PO2). This is in contrast to thecentral chemoreceptorsin themedulla oblongatathat are primarily sensitive to changes in pH and PCO2(a decrease in pH and an increase in PCO2). The carotid body chemoreceptors are also sensitive to pH and PCO2,but only secondarily. More specifically, the sensitivity of carotid body chemoreceptors to decreased PO2is greater when pH is decreased and PCO2is increased.
Impulse rate for carotid bodies is particularly sensitive to changes in arterial PO2 in the range of 60 down to 30 mm Hg, a range in which hemoglobin saturation with oxygen decreases rapidly.[5]
The output of the carotid bodies is low at an oxygenpartial pressureabove about 100mmHg (13,3 kPa) (at normal physiological pH), but below 60mmHg the activity of the type I (glomus) cells increases rapidly due to a decrease in hemoglobin-oxygen saturation below 90%.
Detection
[edit]The mechanism for detecting reductions in PO2has yet to be identified, there may be multiple mechanisms and could vary between species.[6]Hypoxiadetection has been shown to depend upon increasedhydrogen sulfidegeneration produced bycystathionine gamma-lyaseas hypoxia detection is reduced in mice in which this enzyme is knocked out or pharmacologically inhibited. The process of detection involves the interaction of cystathionine gamma-lyase withhemeoxygenase-2and the production ofcarbon monoxide.[7]Yet, some studies show that physiologic concentration of hydrogen sulfide may not be strong enough to trigger such responses.
Other theories suggest it may involve mitochondrial oxygen sensors and the haem-containing cytochromes that undergo reversible one-electron reduction during oxidative-phosphorylation. Haem reversibly binds O2with an affinity similar to that of the carotid body, suggesting that haem containing proteins may have a role in O2,potentially this could be one of the complexes involved in oxidative-phosphorylation. This leads to increases in reactive oxygen species and rises in intracellular Ca2+.However, whether hypoxia leads to an increase or decrease in reactive oxygen species is unknown. The role of reactive oxygen species in hypoxia sensing is also under question.[8]
The oxygen dependent enzyme haem-oxidase has also been put forward as a hypoxia sensor. In normoxia, haem-oxygenase generates carbon monoxide (CO), CO activates the large conductance calcium-activated potassium channel, BK. Falls in CO that occur as a consequence of hypoxia would lead to closure of this potassium channel and this would lead to membrane depolarisation and consequence activation of the carotid body.[9]A role for the "energy sensor" AMP-activated protein kinase (AMPK) has also been proposed in hypoxia sensing. This enzyme is activated during times of net energy usage and metabolic stress, including hypoxia. AMPK has a number of targets and it appears that, in the carotid body, when AMPK is activated by hypoxia, it leads to downstream potassium channel closure of both O2-sentive TASK-like and BK channels[10]
An increased PCO2is detected because the CO2diffusesinto the cell, where it increases the concentration ofcarbonic acidand thusprotons.The precise mechanism of CO2sensing is unknown, however it has been demonstrated that CO2and low pH inhibit a TASK-like potassium conductance, reducing potassium current. This leads to depolarisation of the cell membrane which leads to Ca2+entry, excitation of glomus cells and consequent neurotransmitter release.[11]
Arterialacidosis(eithermetabolicor from alteredPCO2) inhibits acid-base transporters (e.g. Na+-H+) which raiseintracellular pH,and activates transporters (e.g. Cl−-HCO3−) which decrease it. Changes in proton concentration caused by acidosis (or the opposite fromalkalosis) inside the cell stimulates the same pathways involved in PCO2sensing.
Another mechanism is through oxygen sensitive potassium channels. A drop in dissolved oxygen lead to closing of these channels which results in depolarization. This leads to release of the neurotransmitter dopamine in the glossopharyngeal and vagus afferente to the vasomotor area.
Action potential
[edit]The type I (glomus) cells in the carotid (and aortic bodies) are derived from neuroectoderm and are thus electrically excitable. A decrease in oxygen partial pressure, an increase in carbon dioxide partial pressure, and a decrease in arterial pH can all causedepolarizationof thecell membrane,and they affect this by blockingpotassiumcurrents. This reduction in themembrane potentialopensvoltage-gatedcalciumchannels, which causes a rise in intracellular calcium concentration. This causesexocytosisofvesiclescontaining a variety ofneurotransmitters,includingacetylcholine,noradrenaline,dopamine,adenosine,ATP,substance P,andmet-enkephalin.These act onreceptorson the afferent nerve fibres which lie in apposition to the glomus cell to cause an action potential.
Relay
[edit]The feedback from the carotid body is sent to the cardiorespiratory centers in themedulla oblongatavia the afferent branches of theglossopharyngeal nerve.(The efferent fibers of theaortic bodychemoreceptors are relayed by thevagus nerve.) These centers, in turn, regulate breathing and blood pressure, with hypoxia causing an increase in ventilation.
Clinical significance
[edit]
Paraganglioma
[edit]Aparagangliomais a tumor that may involve the carotid body and is usuallybenign.Rarely, a malignantneuroblastomamay originate from the carotid body.
See also
[edit]List of distinct cell types in the adult human body
References
[edit]- ^"Carotid Body and Carotid Sinus -- General Information".Iowa Head and Neck Protocols.medicine.uiowa.edu.Retrieved23 October2019.
- ^Hall, John Edward (20 May 2015).Guyton and Hall textbook of medical physiology(13th ed.). Philadelphia, PA.ISBN978-1-4557-7005-2.OCLC900869748.
{{cite book}}:CS1 maint: location missing publisher (link) - ^Kadasne, D. K. (2009).Kadasne's Textbook of Anatomy(1st ed.). New Delhi: Jaypee Brothers Medical Publishers. p. 916.ISBN978-81-8448-455-7.OCLC682534511.
- ^Gonzalez C, Almaraz L, Obeso A, Rigual R (1994). "Carotid body chemoreceptors: from natural stimuli to sensory discharges".Physiol. Rev.74(4): 829–98.doi:10.1152/physrev.1994.74.4.829.PMID7938227.
- ^Hall, John Edward (20 May 2015).Guyton and Hall textbook of medical physiology(13th ed.). Philadelphia, PA.ISBN978-1-4557-7005-2.OCLC900869748.
{{cite book}}:CS1 maint: location missing publisher (link) - ^Ward JP (2008)."Oxygen sensors in context".Biochim Biophys Acta.1777(1): 1–14.doi:10.1016/j.bbabio.2007.10.010.PMID18036551.
- ^Peng Y-J, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla M.M, Kumar GK, Snyder SH, Prabhakar NR. (2010). H2S mediates O2 sensing in the carotid body PNAS 107 (23) 10719-10724.doi:10.1073/pnas.1005866107
- ^Gonzalez C, Sanz-Alfayate G, Agapito MT, Gomez-Niño A, Rocher A, Obeso A (2002)."Oxygen sensors in context".Respir Physiol Neurobiol.132(1): 17–41.doi:10.1016/S1569-9048(02)00047-2.PMID12126693.S2CID25674998.
- ^Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ (2004)."Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel".Science.306(5704): 2093–7.Bibcode:2004Sci...306.2093W.doi:10.1126/science.1105010.PMID15528406.S2CID41811182.
- ^Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM (2007)."AMP-activated Protein Kinase Mediates Carotid Body Excitation by Hypoxia".J Biol Chem.282(11): 8092–8.doi:10.1074/jbc.M608742200.PMC1832262.PMID17179156.
- ^Buckler KJ, Williams BA, Honore E (2000)."An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells".J. Physiol.525(1): 135–142.doi:10.1111/j.1469-7793.2000.00135.x.PMC2269923.PMID10811732.
