Cathodoluminescence
This article has multiple issues.Please helpimprove itor discuss these issues on thetalk page.(Learn how and when to remove these template messages)
|

Cathodoluminescenceis anopticalandelectromagnetic phenomenonin which electrons impacting on aluminescentmaterial such as aphosphor,cause the emission ofphotonswhich may have wavelengths in thevisible spectrum.A familiar example is the generation of light by an electron beam scanning the phosphor-coated inner surface of the screen of atelevisionthat uses acathode ray tube.Cathodoluminescence is the inverse of thephotoelectric effect,in which electron emission is induced by irradiation with photons.
Origin[edit]
Luminescencein asemiconductorresults when anelectronin theconduction bandrecombines with aholein the valence band. The difference energy (band gap) of this transition can be emitted in form of aphoton.The energy (color) of the photon, and the probability that a photon and not aphononwill be emitted, depends on the material, its purity, and the presence of defects. First, the electron has to be excited from thevalence bandinto theconduction band.In cathodoluminescence, this occurs as the result of an impinging high energy electron beam onto asemiconductor.However, these primary electrons carry far too much energy to directly excite electrons. Instead, the inelastic scattering of the primary electrons in the crystal leads to the emission ofsecondary electrons,Auger electronsandX-rays,which in turn can scatter as well. Such a cascade of scattering events leads to up to 103secondary electrons per incident electron.[1]These secondary electrons can excite valence electrons into the conduction band when they have a kinetic energy about three times theband gapenergy of the material.[2]From there the electron recombines with a hole in the valence band and creates a photon. The excess energy is transferred to phonons and thus heats the lattice. One of the advantages of excitation with an electron beam is that the band gap energy of materials that are investigated is not limited by the energy of the incident light as in the case ofphotoluminescence.Therefore, in cathodoluminescence, the "semiconductor" examined can, in fact, be almost any non-metallic material. In terms ofband structure,classical semiconductors, insulators, ceramics, gemstones, minerals, and glasses can be treated the same way.
Microscopy[edit]

Ingeology,mineralogy,materials scienceandsemiconductorengineering, ascanning electron microscope (SEM)fitted with a cathodoluminescence detector, or an opticalcathodoluminescence microscope,may be used to examine internal structures of semiconductors, rocks,ceramics,glass,etc. in order to get information on the composition, growth and quality of the material.
Optical cathodoluminescence microscope[edit]

Acathodoluminescence(CL)microscopecombines a regular (light optical)microscopewith acathode-ray tube.It is designed to image theluminescencecharacteristics of polished thin sections of solids irradiated by anelectron beam.
Using acathodoluminescencemicroscope, structures withincrystalsor fabrics can be made visible which cannot be seen in normal light conditions. Thus, for example, valuable information on the growth of minerals can be obtained. CL-microscopy is used ingeology,mineralogyandmaterials sciencefor the investigation ofrocks,minerals,volcanic ash,glass,ceramic,concrete,fly ash,etc.
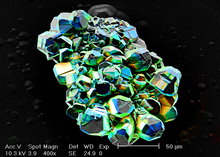
CL color and intensity are dependent on the characteristics of the sample and on the working conditions of theelectron gun.Here,acceleration voltageand beam current of theelectron beamare of major importance. Today, two types of CL microscopes are in use. One is working with a "cold cathode"generating an electron beam by acorona dischargetube, the other one produces a beam using a "hot cathode".Cold-cathode CL microscopes are the simplest and most economical type. Unlike other electron bombardment techniques likeelectron microscopy,cold cathodoluminescence microscopy provides positive ions along with the electrons which neutralize surface charge buildup and eliminate the need for conductive coatings to be applied to the specimens. The "hot cathode" type generates an electron beam by an electron gun with tungsten filament. The advantage of a hot cathode is the precisely controllable high beam intensity allowing to stimulate the emission of light even on weakly luminescing materials (e.g.quartz– see picture). To prevent charging of the sample, the surface must be coated with a conductive layer ofgoldorcarbon.This is usually done by asputter depositiondevice or a carbon coater.
Cathodoluminescence from a scanning electron microscope[edit]


Inscanning electron microscopesa focused beam of electrons impinges on a sample and induces it to emit light that is collected by an optical system, such as an elliptical mirror. From there, afiber opticwill transfer the light out of the microscope where it is separated into its component wavelengths by amonochromatorand is then detected with aphotomultipliertube. By scanning the microscope's beam in an X-Y pattern and measuring the light emitted with the beam at each point, a map of the optical activity of the specimen can be obtained (cathodoluminescence imaging). Instead, by measuring the wavelength dependence for a fixed point or a certain area, the spectral characteristics can be recorded (cathodoluminescence spectroscopy). Furthermore, if the photomultiplier tube is replaced with aCCD camera,an entirespectrumcan be measured at each point of a map (hyperspectral imaging). Moreover, the optical properties of an object can be correlated to structural properties observed with the electron microscope.
The primary advantages to the electron microscope based technique is its spatial resolution. In a scanning electron microscope, the attainable resolution is on the order of a few ten nanometers,[3]while in a (scanning)transmission electron microscope(TEM), nanometer-sized features can be resolved.[4]Additionally, it is possible to perform nanosecond- to picosecond-level time-resolved measurements if the electron beam can be "chopped" into nano- or pico-second pulses by a beam-blanker or with a pulsed electron source. These advanced techniques are useful for examining low-dimensional semiconductor structures, such aquantum wellsorquantum dots.
While an electron microscope with a cathodoluminescence detector provides high magnification, an optical cathodoluminescence microscope benefits from its ability to show actual visible color features directly through the eyepiece. More recently developed systems try to combine both an optical and an electron microscope to take advantage of both these techniques.[5]
Extended applications[edit]
Althoughdirect bandgapsemiconductors such asGaAsorGaNare most easily examined by these techniques, indirect semiconductors such assiliconalso emit weak cathodoluminescence, and can be examined as well. In particular, the luminescence ofdislocatedsilicon is different from intrinsic silicon, and can be used to map defects inintegrated circuits.
Recently, cathodoluminescence performed in electron microscopes is also being used to studysurface plasmon resonancesin metallicnanoparticles.[6]Surfaceplasmonsin metal nanoparticles can absorb and emit light, though the process is different from that in semiconductors. Similarly, cathodoluminescence has been exploited as a probe to map the local density of states of planar dielectricphotonic crystalsand nanostructured photonic materials.[7]
See also[edit]
References[edit]
- ^Mitsui, T; Sekiguchi, T; Fujita, D; Koguchi, N. (2005). "Comparison between electron beam and near-field light on the luminescence excitation of GaAs/AlGaAs semiconductor quantum dots".Jpn. J. Appl. Phys.44(4A): 1820–1824.Bibcode:2005JaJAP..44.1820M.doi:10.1143/JJAP.44.1820.S2CID56031946.
- ^Klein, C. A. (1968). "Bandgap dependence and related features of radiation ionization energies in semiconductors".J. Appl. Phys.39(4): 2029–2038.Bibcode:1968JAP....39.2029K.doi:10.1063/1.1656484.
- ^Lähnemann, J.; Hauswald, C.; Wölz, M.; Jahn, U.; Hanke, M.; Geelhaar, L.; Brandt, O. (2014). "Localization and defects in axial (In,Ga)N/GaN nanowire heterostructures investigated by spatially resolved luminescence spectroscopy".J. Phys. D: Appl. Phys.47(39): 394010.arXiv:1405.1507.Bibcode:2014JPhD...47M4010L.doi:10.1088/0022-3727/47/39/394010.S2CID118314773.
- ^Zagonel; et al. (2011). "Nanometer Scale Spectral Imaging of Quantum Emitters in Nanowires and Its Correlation to Their Atomically Resolved Structure".Nano Letters.11(2): 568–73.arXiv:1209.0953.Bibcode:2011NanoL..11..568Z.doi:10.1021/nl103549t.PMID21182283.S2CID18003378.
- ^"What is Quantitative Cathodoluminescence?".2023-08-23.
- ^García de Abajo, F. J. (2010)."Optical excitations in electron microscopy"(PDF).Reviews of Modern Physics.82(1): 209–275.arXiv:0903.1669.Bibcode:2010RvMP...82..209G.doi:10.1103/RevModPhys.82.209.hdl:10261/79235.S2CID119246090.
- ^Sapienza, R.; Coenen, R.; Renger, J.; Kuttge, M.; van Hulst, N. F.; Polman, A (2012). "Deep-subwavelength imaging of the modal dispersion of light".Nature Materials.11(9): 781–787.Bibcode:2012NatMa..11..781S.doi:10.1038/nmat3402.PMID22902895.S2CID31259521.
Further reading[edit]
- Coenen, T. (2014).Angle-resolved cathodoluminescence nanoscopy(Thesis). University of Amsterdam.hdl:11245/1.417564.
- Electron beams set nanostructures aglow[PDF],E. S. Reich, Nature 493, 143 (2013)
- Lähnemann, J. (2013).Luminescence of group-III-V nanowires containing heterostructures(pdf)(PhD Thesis). Humboldt-Universität zu Berlin.
- Kuttge, M. (2009).Cathodoluminescence plasmon microscopy(pdf)(Thesis). Utrecht University.
- Scanning Cathodoluminescence Microscopy,C. M. Parish and P. E. Russell, inAdvances in Imaging and Electron Physics, V.147,ed. P. W. Hawkes, P. 1 (2007)
- Quick look cathodoluminescence analyses and their impact on the interpretation of carbonate reservoirs. Case study of mid-Jurassic oolitic reservoirs in the Paris BasinArchived2018-09-25 at theWayback Machine,B. Granier and C. Staffelbach (2009)
- Cathodoluminescence Microscopy of Inorganic Solids,,B. G. Yacobi and D. B. Holt, New York, Springer (1990)

