Chromium(III) iodide
| |
| Names | |
|---|---|
| IUPAC name
Chromium(III) iodide
| |
| Other names
Chromium triiodide, chromic iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.614 |
| EC Number |
|
PubChemCID
|
|
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| CrI3 | |
| Molar mass | 432.7095g·mol−1 |
| Appearance | black solid |
| Density | 5.32 g/cm3[1] |
| Melting point | > 600 °C (1,112 °F; 873 K) |
| Soluble | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Chromium(III) iodide,also known as chromium triiodide, is aninorganic compoundwith the formulaCrI3.It is a black solid that is used to prepare other chromium iodides.[2]
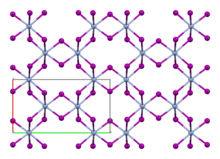
Like theisomorphouschromium(III) chloride(CrCl3), chromium(III) iodide exhibits a cubic-closest packing arrangement in a double-layer crystal lattice. In this structure, chromium exhibitsoctahedral coordination geometry.[3]
Preparation and properties
[edit]Chromium triiodide is prepared by the direct reaction ofchromiummetal with an excess ofiodine.The reaction is conducted at 500 °C:
- 2 Cr + 3 I2→ 2 CrI3
To obtain high purity samples, the product isthermally decomposedat 700 °C tosublimeoutchromium(II) iodide.The diiodide is then reiodinated.[2]
Chromium triiodide is stable in contact withoxygenandmoisture,but at temperatures approaching 200 °C it reacts with oxygen and releases iodine. LikeCrCl3,the triiodide exhibits slow solubility in water owing to the kinetic inertness of Cr(III). Addition of small amounts ofchromous iodideaccelerates thedissolvingprocess.[2]
Chromium triiodide can also be prepared asnanoplatelets[clarification needed]from thealkoxideCr(OC(CH3)(C(CH3)3)2)3.[4][how?]
Chromium triiodide was one of the first materials which was discovered to be amagnetic two-dimensional materialthat has great potentials forspintronicsdevices.[5]
References
[edit]- ^Perry, Dale L. (2011).Handbook of Inorganic Compounds, Second Edition.Boca Raton, Florida: CRC Press. p. 123.ISBN978-1-43981462-8.Retrieved2014-01-10.
- ^abcGregory, N. W.; Handy, L. L. (1957). "Chromium (III) Iodide".Inorganic Syntheses.Vol. 5. pp. 128–130.doi:10.1002/9780470132364.ch34.
{{cite book}}:|journal=ignored (help) - ^Gregory, N. W.; Handy, L. L. (1952). "Structural Properties of Chromium(III) Iodide and Some Chromium(III) Mixed Halides".J. Am. Chem. Soc.74(4): 891–893.doi:10.1021/ja01124a009.
- ^De Siena, Michael C.; Creutz, Sidney E.; Regan, Annie; Malinowski, Paul; Jiang, Qianni; Kluherz, Kyle T.; Zhu, Guomin; Lin, Zhong; De Yoreo, James J.; Xu, Xiaodong; Chu, Jiun-Haw (2020-03-11)."Two-Dimensional van der Waals Nanoplatelets with Robust Ferromagnetism".Nano Letters.20(3): 2100–2106.arXiv:2001.04594.Bibcode:2020NanoL..20.2100D.doi:10.1021/acs.nanolett.0c00102.ISSN1530-6984.PMID32031382.S2CID210473134.
- ^Huang, B.; et al. (2017). "Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit".Nature.546(7657): 270–273.arXiv:1703.05892.Bibcode:2017Natur.546..270H.doi:10.1038/nature22391.PMID28593970.S2CID4456526.
