Conserved sequence

Sequences are theamino acidsfor residues 120-180 of the proteins. Residues that are conserved across all sequences are highlighted in grey. Below each site (i.e., position) of the protein sequence alignment is a key denoting conserved sites (*), sites withconservative replacements(:), sites with semi-conservative replacements (.), and sites withnon-conservative replacements( ).[1]
Inevolutionary biology,conserved sequencesare identical or similarsequencesinnucleic acids(DNAandRNA) orproteinsacross species (orthologous sequences), or within agenome(paralogous sequences), or between donor and receptor taxa (xenologous sequences). Conservation indicates that a sequence has been maintained bynatural selection.
A highly conserved sequence is one that has remained relatively unchanged far back up thephylogenetic tree,and hence far back ingeological time.Examples of highly conserved sequences include theRNA componentsofribosomespresent in alldomainsof life, thehomeoboxsequences widespread amongsteukaryotes,and thetmRNAinbacteria.The study of sequence conservation overlaps with the fields ofgenomics,proteomics,evolutionary biology,phylogenetics,bioinformaticsandmathematics.
History[edit]
The discovery of the role ofDNAinheredity,and observations byFrederick Sangerof variation between animalinsulinsin 1949,[2]prompted early molecular biologists to studytaxonomyfrom a molecular perspective.[3][4]Studies in the 1960s usedDNA hybridizationand protein cross-reactivity techniques to measure similarity between knownorthologousproteins, such ashemoglobin[5]andcytochrome c.[6]In 1965,Émile ZuckerkandlandLinus Paulingintroduced the concept of themolecular clock,[7]proposing that steady rates of amino acid replacement could be used to estimate the time since two organismsdiverged.While initial phylogenies closely matched thefossil record,observations that some genes appeared to evolve at different rates led to the development of theories ofmolecular evolution.[3][4]Margaret Dayhoff's1966 comparison offerredoxinsequences showed thatnatural selectionwould act to conserve and optimise protein sequences essential to life.[8]
Mechanisms[edit]
Over many generations, nucleic acid sequences in thegenomeof anevolutionary lineagecan gradually change over time due to random mutations anddeletions.[9][10]Sequences may also recombine or be deleted due tochromosomal rearrangements.Conserved sequences are sequences which persist in the genome despite such forces, and have slower rates of mutation than the background mutation rate.[11]
Conservation can occur incodingandnon-codingnucleic acid sequences. Highly conserved DNA sequences are thought to have functional value, although the role for many highly conservednon-coding DNAsequences is poorly understood.[12][13]The extent to which a sequence is conserved can be affected by varyingselection pressures,itsrobustnessto mutation,population sizeandgenetic drift.Many functional sequences are alsomodular,containing regions which may be subject to independentselection pressures,such asprotein domains.[14]
Coding sequence[edit]
In coding sequences, the nucleic acid and amino acid sequence may be conserved to different extents, as the degeneracy of thegenetic codemeans thatsynonymous mutationsin a coding sequence do not affect the amino acid sequence of its protein product.[15]
Amino acid sequences can be conserved to maintain thestructureor function of a protein or domain. Conserved proteins undergo feweramino acid replacements,or are more likely tosubstitute amino acids with similar biochemical properties.[16]Within a sequence, amino acids that are important forfolding,structural stability, or that form abinding sitemay be more highly conserved.[17][18]
The nucleic acid sequence of a protein coding gene may also be conserved by other selective pressures. Thecodon usage biasin some organisms may restrict the types of synonymous mutations in a sequence. Nucleic acid sequences that causesecondary structurein the mRNA of a coding gene may be selected against, as some structures may negatively affect translation, or conserved where the mRNA also acts as a functional non-coding RNA.[19][20]
Non-coding[edit]
Non-coding sequences important forgene regulation,such as the binding or recognition sites ofribosomesandtranscription factors,may be conserved within a genome. For example, thepromoterof a conserved gene oroperonmay also be conserved. As with proteins, nucleic acids that are important for the structure and function ofnon-coding RNA(ncRNA) can also be conserved. However, sequence conservation in ncRNAs is generally poor compared to protein-coding sequences, andbase pairsthat contribute to structure or function are often conserved instead.[21][22]
Identification[edit]
Conserved sequences are typically identified bybioinformaticsapproaches based onsequence alignment.Advances inhigh-throughput DNA sequencingandprotein mass spectrometryhas substantially increased the availability of protein sequences and whole genomes for comparison since the early 2000s.[23][24]
Homology search[edit]
Conserved sequences may be identified byhomologysearch, using tools such asBLAST,HMMER,OrthologR,[25]and Infernal.[26]Homology search tools may take an individual nucleic acid or protein sequence as input, or use statistical models generated frommultiple sequence alignmentsof known related sequences. Statistical models such asprofile-HMMs,and RNA covariance models which also incorporate structural information,[27]can be helpful when searching for more distantly related sequences. Input sequences are then aligned against a database of sequences from related individuals or other species. The resulting alignments are then scored based on the number of matching amino acids or bases, and the number of gaps or deletions generated by the alignment. Acceptable conservative substitutions may be identified using substitution matrices such asPAMandBLOSUM.Highly scoring alignments are assumed to be from homologous sequences. The conservation of a sequence may then be inferred by detection of highly similar homologs over a broad phylogenetic range.[28]
Multiple sequence alignment[edit]
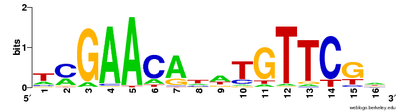
Multiple sequence alignments can be used to visualise conserved sequences. TheCLUSTALformat includes a plain-text key to annotate conserved columns of the alignment, denoting conserved sequence (*), conservative mutations (:), semi-conservative mutations (.), and non-conservative mutations ( )[30]Sequence logos can also show conserved sequence by representing the proportions of characters at each point in the alignment by height.[29]
Genome alignment[edit]

Whole genome alignments (WGAs) may also be used to identify highly conserved regions across species. Currently the accuracy andscalabilityof WGA tools remains limited due to the computational complexity of dealing with rearrangements, repeat regions and the large size of many eukaryotic genomes.[32]However, WGAs of 30 or more closely related bacteria (prokaryotes) are now increasingly feasible.[33][34]
Scoring systems[edit]
Other approaches use measurements of conservation based onstatistical teststhat attempt to identify sequences which mutate differently to an expected background (neutral) mutation rate.
The GERP (Genomic Evolutionary Rate Profiling) framework scores conservation of genetic sequences across species. This approach estimates the rate of neutral mutation in a set of species from a multiple sequence alignment, and then identifies regions of the sequence that exhibit fewer mutations than expected. These regions are then assigned scores based on the difference between the observed mutation rate and expected background mutation rate. A high GERP score then indicates a highly conserved sequence.[35][36]
LIST[37] [38](Local Identity and Shared Taxa) is based on the assumption that variations observed in species closely related to human are more significant when assessing conservation compared to those in distantly related species. Thus, LIST utilizes the local alignment identity around each position to identify relevant sequences in the multiple sequence alignment (MSA) and then it estimates conservation based on the taxonomy distances of these sequences to human. Unlike other tools, LIST ignores the count/frequency of variations in the MSA.
Aminode[39]combines multiple alignments with phylogenetic analysis to analyze changes in homologous proteins and produce a plot that indicates the local rates of evolutionary changes. This approach identifies the Evolutionarily Constrained Regions in a protein, which are segments that are subject topurifying selectionand are typically critical for normal protein function.
Other approaches such as PhyloP and PhyloHMM incorporatestatistical phylogeneticsmethods to compareprobability distributionsof substitution rates, which allows the detection of both conservation and accelerated mutation. First, a background probability distribution is generated of the number of substitutions expected to occur for a column in a multiple sequence alignment, based on aphylogenetic tree.The estimated evolutionary relationships between the species of interest are used to calculate the significance of any substitutions (i.e. a substitution between two closely related species may be less likely to occur than distantly related ones, and therefore more significant). To detect conservation, a probability distribution is calculated for a subset of the multiple sequence alignment, and compared to the background distribution using a statistical test such as alikelihood-ratio testorscore test.P-valuesgenerated from comparing the two distributions are then used to identify conserved regions. PhyloHMM useshidden Markov modelsto generate probability distributions. The PhyloP software package compares probability distributions using alikelihood-ratio testorscore test,as well as using a GERP-like scoring system.[40][41][42]
Extreme conservation[edit]
Ultra-conserved elements[edit]
Ultra-conserved elementsor UCEs are sequences that are highly similar or identical across multipletaxonomic groupings.These were first discovered invertebrates,[43]and have subsequently been identified within widely-differing taxa.[44]While the origin and function of UCEs are poorly understood,[45]they have been used to investigate deep-time divergences inamniotes,[46]insects,[47]and betweenanimalsandplants.[48]
Universally conserved genes[edit]
The most highly conserved genes are those that can be found in all organisms. These consist mainly of thencRNAsand proteins required fortranscriptionandtranslation,which are assumed to have been conserved from thelast universal common ancestorof all life.[49]
Genes or gene families that have been found to be universally conserved includeGTP-binding elongation factors,Methionine aminopeptidase 2,Serine hydroxymethyltransferase,andATP transporters.[50]Components of the transcription machinery, such asRNA polymeraseandhelicases,and of the translation machinery, such asribosomal RNAs,tRNAsandribosomal proteinsare also universally conserved.[51]
Applications[edit]
Phylogenetics and taxonomy[edit]
Sets of conserved sequences are often used for generatingphylogenetic trees,as it can be assumed that organisms with similar sequences are closely related.[52]The choice of sequences may vary depending on the taxonomic scope of the study. For example, the most highly conserved genes such as the 16S RNA and other ribosomal sequences are useful for reconstructing deep phylogenetic relationships and identifying bacterialphylainmetagenomicsstudies.[53][54]Sequences that are conserved within acladebut undergo some mutations, such ashousekeeping genes,can be used to study species relationships.[55][56][57]Theinternal transcribed spacer(ITS) region, which is required for spacing conserved rRNA genes but undergoes rapid evolution, is commonly used to classifyfungiand strains of rapidly evolving bacteria.[58][59][60][61]
Medical research[edit]
As highly conserved sequences often have important biological functions, they can be useful a starting point for identifying the cause ofgenetic diseases.Manycongenital metabolic disordersandLysosomal storage diseasesare the result of changes to individual conserved genes, resulting in missing or faulty enzymes that are the underlying cause of the symptoms of the disease. Genetic diseases may be predicted by identifying sequences that are conserved between humans and lab organisms such asmice[62]orfruit flies,[63]and studying the effects ofknock-outsof these genes.[64]Genome-wide association studiescan also be used to identify variation in conserved sequences associated with disease or health outcomes. More than two dozen novel potential susceptibility loci have been discovered for Alzehimer's disease.[65][66]
Functional annotation[edit]
Identifying conserved sequences can be used to discover and predict functional sequences such as genes.[67]Conserved sequences with a known function, such as protein domains, can also be used to predict the function of a sequence. Databases of conserved protein domains such asPfamand theConserved Domain Databasecan be used to annotate functional domains in predicted protein coding genes.[68]
See also[edit]
- Evolutionary developmental biology
- NAPP (database)
- Segregating site
- Sequence alignment
- Sequence alignment software
- UCbase
- Ultra-conserved element
References[edit]
- ^"Clustal FAQ #Symbols".Clustal.Archived fromthe originalon 24 October 2016.Retrieved8 December2014.
- ^Sanger, F. (24 September 1949)."Species Differences in Insulins".Nature.164(4169): 529.Bibcode:1949Natur.164..529S.doi:10.1038/164529a0.PMID18141620.S2CID4067991.
- ^abMarmur, J; Falkow, S; Mandel, M (October 1963). "New Approaches to Bacterial Taxonomy".Annual Review of Microbiology.17(1): 329–372.doi:10.1146/annurev.mi.17.100163.001553.PMID14147455.
- ^abPace, N. R.; Sapp, J.; Goldenfeld, N. (17 January 2012)."Phylogeny and beyond: Scientific, historical, and conceptual significance of the first tree of life".Proceedings of the National Academy of Sciences.109(4): 1011–1018.Bibcode:2012PNAS..109.1011P.doi:10.1073/pnas.1109716109.PMC3268332.PMID22308526.
- ^Zuckerlandl, Emile;Pauling, Linus B.(1962). "Molecular disease, evolution, and genetic heterogeneity".Horizons in Biochemistry:189–225.
- ^Margoliash, E (October 1963)."Primary Structure and Evolution of Cytochrome C".Proceedings of the National Academy of Sciences.50(4): 672–679.Bibcode:1963PNAS...50..672M.doi:10.1073/pnas.50.4.672.PMC221244.PMID14077496.
- ^Zuckerkandl, E; Pauling, LB (1965). "Evolutionary Divergence and Convergence in Proteins".Evolving Genes and And Proteins:96–166.doi:10.1016/B978-1-4832-2734-4.50017-6.ISBN9781483227344.
- ^Eck, R. V.; Dayhoff, M. O. (15 April 1966). "Evolution of the Structure of Ferredoxin Based on Living Relics of Primitive Amino Acid Sequences".Science.152(3720): 363–366.Bibcode:1966Sci...152..363E.doi:10.1126/science.152.3720.363.PMID17775169.S2CID23208558.
- ^Kimura, M (17 February 1968). "Evolutionary Rate at the Molecular Level".Nature.217(5129): 624–626.Bibcode:1968Natur.217..624K.doi:10.1038/217624a0.PMID5637732.S2CID4161261.
- ^King, J. L.; Jukes, T. H. (16 May 1969). "Non-Darwinian Evolution".Science.164(3881): 788–798.Bibcode:1969Sci...164..788L.doi:10.1126/science.164.3881.788.PMID5767777.
- ^Kimura, M; Ohta, T (1974)."On Some Principles Governing Molecular Evolution".Proc Natl Acad Sci USA.71(7): 2848–2852.Bibcode:1974PNAS...71.2848K.doi:10.1073/pnas.71.7.2848.PMC388569.PMID4527913.
- ^Asthana, Saurabh; Roytberg, Mikhail; Stamatoyannopoulos, John; Sunyaev, Shamil (28 December 2007). Brudno, Michael (ed.)."Analysis of Sequence Conservation at Nucleotide Resolution".PLOS Computational Biology.3(12): e254.Bibcode:2007PLSCB...3..254A.doi:10.1371/journal.pcbi.0030254.ISSN1553-7358.PMC2230682.PMID18166073.
- ^Cooper, G. M.; Brown, C. D. (1 February 2008)."Qualifying the relationship between sequence conservation and molecular function".Genome Research.18(2): 201–205.doi:10.1101/gr.7205808.ISSN1088-9051.PMID18245453.
- ^Gilson, Amy I.; Marshall-Christensen, Ahmee; Choi, Jeong-Mo; Shakhnovich, Eugene I. (2017)."The Role of Evolutionary Selection in the Dynamics of Protein Structure Evolution".Biophysical Journal.112(7): 1350–1365.arXiv:1606.05802.Bibcode:2017BpJ...112.1350G.doi:10.1016/j.bpj.2017.02.029.PMC5390048.PMID28402878.
- ^Hunt, Ryan C.; Simhadri, Vijaya L.; Iandoli, Matthew; Sauna, Zuben E.; Kimchi-Sarfaty, Chava (2014). "Exposing synonymous mutations".Trends in Genetics.30(7): 308–321.doi:10.1016/j.tig.2014.04.006.PMID24954581.
- ^Zhang, Jianzhi (2000)."Rates of Conservative and Radical Nonsynonymous Nucleotide Substitutions in Mammalian Nuclear Genes".Journal of Molecular Evolution.50(1): 56–68.Bibcode:2000JMolE..50...56Z.doi:10.1007/s002399910007.ISSN0022-2844.PMID10654260.S2CID15248867.
- ^Sousounis, Konstantinos; Haney, Carl E; Cao, Jin; Sunchu, Bharath; Tsonis, Panagiotis A (2012)."Conservation of the three-dimensional structure in non-homologous or unrelated proteins".Human Genomics.6(1): 10.doi:10.1186/1479-7364-6-10.ISSN1479-7364.PMC3500211.PMID23244440.
- ^Kairys, Visvaldas; Fernandes, Miguel X. (2007)."SitCon: Binding site residue conservation visualization and protein sequence-to-function tool".International Journal of Quantum Chemistry.107(11): 2100–2110.Bibcode:2007IJQC..107.2100K.doi:10.1002/qua.21396.hdl:10400.13/5004.ISSN0020-7608.
- ^Chamary, JV; Hurst, Laurence D (2005)."Evidence for selection on synonymous mutations affecting stability of mRNA secondary structure in mammals".Genome Biology.6(9): R75.doi:10.1186/gb-2005-6-9-r75.PMC1242210.PMID16168082.
- ^Wadler, C. S.; Vanderpool, C. K. (27 November 2007)."A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide".Proceedings of the National Academy of Sciences.104(51): 20454–20459.Bibcode:2007PNAS..10420454W.doi:10.1073/pnas.0708102104.PMC2154452.PMID18042713.
- ^Johnsson, Per; Lipovich, Leonard; Grandér, Dan; Morris, Kevin V. (March 2014)."Evolutionary conservation of long non-coding RNAs; sequence, structure, function".Biochimica et Biophysica Acta (BBA) - General Subjects.1840(3): 1063–1071.doi:10.1016/j.bbagen.2013.10.035.PMC3909678.PMID24184936.
- ^Freyhult, E. K.; Bollback, J. P.; Gardner, P. P. (6 December 2006)."Exploring genomic dark matter: A critical assessment of the performance of homology search methods on noncoding RNA".Genome Research.17(1): 117–125.doi:10.1101/gr.5890907.PMC1716261.PMID17151342.
- ^Margulies, E. H. (1 December 2003)."Identification and Characterization of Multi-Species Conserved Sequences".Genome Research.13(12): 2507–2518.doi:10.1101/gr.1602203.ISSN1088-9051.PMC403793.PMID14656959.
- ^Edwards, John R.; Ruparel, Hameer; Ju, Jingyue (2005). "Mass-spectrometry DNA sequencing".Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis.573(1–2): 3–12.doi:10.1016/j.mrfmmm.2004.07.021.PMID15829234.
- ^Drost, Hajk-Georg; Gabel, Alexander; Grosse, Ivo; Quint, Marcel (1 May 2015)."Evidence for Active Maintenance of Phylotranscriptomic Hourglass Patterns in Animal and Plant Embryogenesis".Molecular Biology and Evolution.32(5): 1221–1231.doi:10.1093/molbev/msv012.ISSN0737-4038.PMC4408408.PMID25631928.
- ^Nawrocki, E. P.; Eddy, S. R. (4 September 2013)."Infernal 1.1: 100-fold faster RNA homology searches".Bioinformatics.29(22): 2933–2935.doi:10.1093/bioinformatics/btt509.PMC3810854.PMID24008419.
- ^Eddy, SR; Durbin, R (11 June 1994)."RNA sequence analysis using covariance models".Nucleic Acids Research.22(11): 2079–88.doi:10.1093/nar/22.11.2079.PMC308124.PMID8029015.
- ^Trivedi, Rakesh; Nagarajaram, Hampapathalu Adimurthy (2020)."Substitution scoring matrices for proteins - An overview".Protein Science.29(11): 2150–2163.doi:10.1002/pro.3954.ISSN0961-8368.PMC7586916.PMID32954566.
- ^ab"Weblogo".UC Berkeley.Retrieved30 December2017.
- ^"Clustal FAQ #Symbols".Clustal.Archived fromthe originalon 24 October 2016.Retrieved8 December2014.
- ^"ECR Browser".ECR Browser.Retrieved9 January2018.
- ^Earl, Dent; Nguyen, Ngan; Hickey, Glenn; Harris, Robert S.; Fitzgerald, Stephen; Beal, Kathryn; Seledtsov, Igor; Molodtsov, Vladimir; Raney, Brian J.; Clawson, Hiram; Kim, Jaebum; Kemena, Carsten; Chang, Jia-Ming; Erb, Ionas; Poliakov, Alexander; Hou, Minmei; Herrero, Javier; Kent, William James; Solovyev, Victor; Darling, Aaron E.; Ma, Jian; Notredame, Cedric; Brudno, Michael; Dubchak, Inna; Haussler, David; Paten, Benedict (December 2014)."Alignathon: a competitive assessment of whole-genome alignment methods".Genome Research.24(12): 2077–2089.doi:10.1101/gr.174920.114.PMC4248324.PMID25273068.
- ^Rouli, L.; Merhej, V.; Fournier, P.-E.; Raoult, D. (September 2015)."The bacterial pangenome as a new tool for analysing pathogenic bacteria".New Microbes and New Infections.7:72–85.doi:10.1016/j.nmni.2015.06.005.PMC4552756.PMID26442149.
- ^Méric, Guillaume; Yahara, Koji; Mageiros, Leonardos; Pascoe, Ben; Maiden, Martin C. J.; Jolley, Keith A.; Sheppard, Samuel K.; Bereswill, Stefan (27 March 2014)."A Reference Pan-Genome Approach to Comparative Bacterial Genomics: Identification of Novel Epidemiological Markers in Pathogenic Campylobacter".PLOS ONE.9(3): e92798.Bibcode:2014PLoSO...992798M.doi:10.1371/journal.pone.0092798.PMC3968026.PMID24676150.
- ^Cooper, G. M. (17 June 2005)."Distribution and intensity of constraint in mammalian genomic sequence".Genome Research.15(7): 901–913.doi:10.1101/gr.3577405.PMC1172034.PMID15965027.
- ^"Sidow Lab - GERP".
- ^Nawar Malhis; Steven J. M. Jones; Jörg Gsponer (2019)."Improved measures for evolutionary conservation that exploit taxonomy distances".Nature Communications.10(1): 1556.Bibcode:2019NatCo..10.1556M.doi:10.1038/s41467-019-09583-2.PMC6450959.PMID30952844.
- ^Nawar Malhis; Matthew Jacobson; Steven J. M. Jones; Jörg Gsponer (2020)."LIST-S2: Taxonomy Based Sorting of Deleterious Missense Mutations Across Species".Nucleic Acids Research.48(W1): W154–W161.doi:10.1093/nar/gkaa288.PMC7319545.PMID32352516.
- ^Chang KT, Guo J, di Ronza A, Sardiello M (January 2018)."Aminode: Identification of Evolutionary Constraints in the Human Proteome".Sci. Rep.8(1): 1357.Bibcode:2018NatSR...8.1357C.doi:10.1038/s41598-018-19744-w.PMC5778061.PMID29358731.
- ^Pollard, K. S.; Hubisz, M. J.; Rosenbloom, K. R.; Siepel, A. (26 October 2009)."Detection of nonneutral substitution rates on mammalian phylogenies".Genome Research.20(1): 110–121.doi:10.1101/gr.097857.109.PMC2798823.PMID19858363.
- ^"PHAST: Home".
- ^Fan, Xiaodan; Zhu, Jun; Schadt, Eric E; Liu, Jun S (2007)."Statistical power of phylo-HMM for evolutionarily conserved element detection".BMC Bioinformatics.8(1): 374.doi:10.1186/1471-2105-8-374.PMC2194792.PMID17919331.
- ^Bejerano, G. (28 May 2004). "Ultraconserved Elements in the Human Genome".Science.304(5675): 1321–1325.Bibcode:2004Sci...304.1321B.CiteSeerX10.1.1.380.9305.doi:10.1126/science.1098119.PMID15131266.S2CID2790337.
- ^Siepel, A. (1 August 2005)."Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes".Genome Research.15(8): 1034–1050.doi:10.1101/gr.3715005.PMC1182216.PMID16024819.
- ^Harmston, N.; Baresic, A.; Lenhard, B. (11 November 2013)."The mystery of extreme non-coding conservation".Philosophical Transactions of the Royal Society B: Biological Sciences.368(1632): 20130021.doi:10.1098/rstb.2013.0021.PMC3826495.PMID24218634.
- ^Faircloth, B. C.; McCormack, J. E.; Crawford, N. G.; Harvey, M. G.; Brumfield, R. T.; Glenn, T. C. (9 January 2012)."Ultraconserved Elements Anchor Thousands of Genetic Markers Spanning Multiple Evolutionary Timescales".Systematic Biology.61(5): 717–726.doi:10.1093/sysbio/sys004.PMID22232343.
- ^Faircloth, Brant C.; Branstetter, Michael G.; White, Noor D.; Brady, Seán G. (May 2015)."Target enrichment of ultraconserved elements from arthropods provides a genomic perspective on relationships among Hymenoptera".Molecular Ecology Resources.15(3): 489–501.doi:10.1111/1755-0998.12328.PMC4407909.PMID25207863.
- ^Reneker, J.; Lyons, E.; Conant, G. C.; Pires, J. C.; Freeling, M.; Shyu, C.-R.; Korkin, D. (10 April 2012)."Long identical multispecies elements in plant and animal genomes".Proceedings of the National Academy of Sciences.109(19): E1183–E1191.doi:10.1073/pnas.1121356109.PMC3358895.PMID22496592.
- ^Isenbarger, Thomas A.; Carr, Christopher E.; Johnson, Sarah Stewart; Finney, Michael; Church, George M.; Gilbert, Walter; Zuber, Maria T.; Ruvkun, Gary (14 October 2008). "The Most Conserved Genome Segments for Life Detection on Earth and Other Planets".Origins of Life and Evolution of Biospheres.38(6): 517–533.Bibcode:2008OLEB...38..517I.doi:10.1007/s11084-008-9148-z.PMID18853276.S2CID15707806.
- ^Harris, J. K. (12 February 2003)."The Genetic Core of the Universal Ancestor".Genome Research.13(3): 407–412.doi:10.1101/gr.652803.PMC430263.PMID12618371.
- ^Ban, Nenad; Beckmann, Roland; Cate, Jamie HD; Dinman, Jonathan D; Dragon, François; Ellis, Steven R; Lafontaine, Denis LJ; Lindahl, Lasse; Liljas, Anders; Lipton, Jeffrey M; McAlear, Michael A; Moore, Peter B; Noller, Harry F; Ortega, Joaquin; Panse, Vikram Govind; Ramakrishnan, V; Spahn, Christian MT; Steitz, Thomas A; Tchorzewski, Marek; Tollervey, David; Warren, Alan J; Williamson, James R; Wilson, Daniel; Yonath, Ada; Yusupov, Marat (February 2014)."A new system for naming ribosomal proteins".Current Opinion in Structural Biology.24:165–169.doi:10.1016/j.sbi.2014.01.002.PMC4358319.PMID24524803.
- ^Gadagkar, Sudhindra R.; Rosenberg, Michael S.; Kumar, Sudhir (15 January 2005)."Inferring species phylogenies from multiple genes: Concatenated sequence tree versus consensus gene tree".Journal of Experimental Zoology Part B: Molecular and Developmental Evolution.304B(1): 64–74.Bibcode:2005JEZB..304...64G.doi:10.1002/jez.b.21026.PMID15593277.
- ^Ludwig, W; Schleifer, KH (October 1994)."Bacterial phylogeny based on 16S and 23S rRNA sequence analysis".FEMS Microbiology Reviews.15(2–3): 155–73.doi:10.1111/j.1574-6976.1994.tb00132.x.PMID7524576.
- ^Hug, Laura A.; Baker, Brett J.; Anantharaman, Karthik; Brown, Christopher T.; Probst, Alexander J.; Castelle, Cindy J.; Butterfield, Cristina N.; Hernsdorf, Alex W.; Amano, Yuki; Ise, Kotaro; Suzuki, Yohey; Dudek, Natasha; Relman, David A.; Finstad, Kari M.; Amundson, Ronald; Thomas, Brian C.; Banfield, Jillian F. (11 April 2016)."A new view of the tree of life".Nature Microbiology.1(5): 16048.doi:10.1038/nmicrobiol.2016.48.PMID27572647.
- ^Zhang, Liqing; Li, Wen-Hsiung (February 2004)."Mammalian Housekeeping Genes Evolve More Slowly than Tissue-Specific Genes".Molecular Biology and Evolution.21(2): 236–239.doi:10.1093/molbev/msh010.PMID14595094.
- ^Clermont, O.; Bonacorsi, S.; Bingen, E. (1 October 2000)."Rapid and Simple Determination of the Escherichia coli Phylogenetic Group".Applied and Environmental Microbiology.66(10): 4555–4558.Bibcode:2000ApEnM..66.4555C.doi:10.1128/AEM.66.10.4555-4558.2000.PMC92342.PMID11010916.
- ^Kullberg, Morgan; Nilsson, Maria A.; Arnason, Ulfur; Harley, Eric H.; Janke, Axel (August 2006)."Housekeeping Genes for Phylogenetic Analysis of Eutherian Relationships".Molecular Biology and Evolution.23(8): 1493–1503.doi:10.1093/molbev/msl027.PMID16751257.
- ^Schoch, C. L.; Seifert, K. A.; Huhndorf, S.; Robert, V.; Spouge, J. L.; Levesque, C. A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P. W.; Miller, A. N.; Wingfield, M. J.; Aime, M. C.; An, K.-D.; Bai, F.-Y.; Barreto, R. W.; Begerow, D.; Bergeron, M.-J.; Blackwell, M.; Boekhout, T.; Bogale, M.; Boonyuen, N.; Burgaz, A. R.; Buyck, B.; Cai, L.; Cai, Q.; Cardinali, G.; Chaverri, P.; Coppins, B. J.; Crespo, A.; Cubas, P.; Cummings, C.; Damm, U.; de Beer, Z. W.; de Hoog, G. S.; Del-Prado, R.; Dentinger, B.; Dieguez-Uribeondo, J.; Divakar, P. K.; Douglas, B.; Duenas, M.; Duong, T. A.; Eberhardt, U.; Edwards, J. E.; Elshahed, M. S.; Fliegerova, K.; Furtado, M.; Garcia, M. A.; Ge, Z.-W.; Griffith, G. W.; Griffiths, K.; Groenewald, J. Z.; Groenewald, M.; Grube, M.; Gryzenhout, M.; Guo, L.-D.; Hagen, F.; Hambleton, S.; Hamelin, R. C.; Hansen, K.; Harrold, P.; Heller, G.; Herrera, C.; Hirayama, K.; Hirooka, Y.; Ho, H.-M.; Hoffmann, K.; Hofstetter, V.; Hognabba, F.; Hollingsworth, P. M.; Hong, S.-B.; Hosaka, K.; Houbraken, J.; Hughes, K.; Huhtinen, S.; Hyde, K. D.; James, T.; Johnson, E. M.; Johnson, J. E.; Johnston, P. R.; Jones, E. B. G.; Kelly, L. J.; Kirk, P. M.; Knapp, D. G.; Koljalg, U.; Kovacs, G. M.; Kurtzman, C. P.; Landvik, S.; Leavitt, S. D.; Liggenstoffer, A. S.; Liimatainen, K.; Lombard, L.; Luangsa-ard, J. J.; Lumbsch, H. T.; Maganti, H.; Maharachchikumbura, S. S. N.; Martin, M. P.; May, T. W.; McTaggart, A. R.; Methven, A. S.; Meyer, W.; Moncalvo, J.-M.; Mongkolsamrit, S.; Nagy, L. G.; Nilsson, R. H.; Niskanen, T.; Nyilasi, I.; Okada, G.; Okane, I.; Olariaga, I.; Otte, J.; Papp, T.; Park, D.; Petkovits, T.; Pino-Bodas, R.; Quaedvlieg, W.; Raja, H. A.; Redecker, D.; Rintoul, T. L.; Ruibal, C.; Sarmiento-Ramirez, J. M.; Schmitt, I.; Schussler, A.; Shearer, C.; Sotome, K.; Stefani, F. O. P.; Stenroos, S.; Stielow, B.; Stockinger, H.; Suetrong, S.; Suh, S.-O.; Sung, G.-H.; Suzuki, M.; Tanaka, K.; Tedersoo, L.; Telleria, M. T.; Tretter, E.; Untereiner, W. A.; Urbina, H.; Vagvolgyi, C.; Vialle, A.; Vu, T. D.; Walther, G.; Wang, Q.-M.; Wang, Y.; Weir, B. S.; Weiss, M.; White, M. M.; Xu, J.; Yahr, R.; Yang, Z. L.; Yurkov, A.; Zamora, J.-C.; Zhang, N.; Zhuang, W.-Y.; Schindel, D. (27 March 2012)."Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi".Proceedings of the National Academy of Sciences.109(16): 6241–6246.doi:10.1073/pnas.1117018109.PMC3341068.PMID22454494.
- ^Man, S. M.; Kaakoush, N. O.; Octavia, S.; Mitchell, H. (26 March 2010)."The Internal Transcribed Spacer Region, a New Tool for Use in Species Differentiation and Delineation of Systematic Relationships within the Campylobacter Genus".Applied and Environmental Microbiology.76(10): 3071–3081.Bibcode:2010ApEnM..76.3071M.doi:10.1128/AEM.02551-09.PMC2869123.PMID20348308.
- ^Ranjard, L.; Poly, F.; Lata, J.-C.; Mougel, C.; Thioulouse, J.; Nazaret, S. (1 October 2001)."Characterization of Bacterial and Fungal Soil Communities by Automated Ribosomal Intergenic Spacer Analysis Fingerprints: Biological and Methodological Variability".Applied and Environmental Microbiology.67(10): 4479–4487.Bibcode:2001ApEnM..67.4479R.doi:10.1128/AEM.67.10.4479-4487.2001.PMC93193.PMID11571146.
- ^Bidet, Philippe; Barbut, Frédéric; Lalande, Valérie; Burghoffer, Béatrice; Petit, Jean-Claude (June 1999)."Development of a new PCR-ribotyping method for based on ribosomal RNA gene sequencing".FEMS Microbiology Letters.175(2): 261–266.doi:10.1111/j.1574-6968.1999.tb13629.x.PMID10386377.
- ^Ala, Ugo; Piro, Rosario Michael; Grassi, Elena; Damasco, Christian; Silengo, Lorenzo; Oti, Martin; Provero, Paolo; Di Cunto, Ferdinando; Tucker-Kellogg, Greg (28 March 2008)."Prediction of Human Disease Genes by Human-Mouse Conserved Coexpression Analysis".PLOS Computational Biology.4(3): e1000043.Bibcode:2008PLSCB...4E0043A.doi:10.1371/journal.pcbi.1000043.PMC2268251.PMID18369433.
- ^Pandey, U. B.; Nichols, C. D. (17 March 2011)."Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery".Pharmacological Reviews.63(2): 411–436.doi:10.1124/pr.110.003293.PMC3082451.PMID21415126.
- ^Huang, Hui; Winter, Eitan E; Wang, Huajun; Weinstock, Keith G; Xing, Heming; Goodstadt, Leo; Stenson, Peter D; Cooper, David N; Smith, Douglas; Albà, M Mar; Ponting, Chris P; Fechtel, Kim (2004)."Evolutionary conservation and selection of human disease gene orthologs in the rat and mouse genomes".Genome Biology.5(7): R47.doi:10.1186/gb-2004-5-7-r47.PMC463309.PMID15239832.
- ^Ge, Dongliang; Fellay, Jacques; Thompson, Alexander J.; Simon, Jason S.; Shianna, Kevin V.; Urban, Thomas J.; Heinzen, Erin L.; Qiu, Ping; Bertelsen, Arthur H.; Muir, Andrew J.; Sulkowski, Mark; McHutchison, John G.; Goldstein, David B. (16 August 2009). "Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance".Nature.461(7262): 399–401.Bibcode:2009Natur.461..399G.doi:10.1038/nature08309.PMID19684573.S2CID1707096.
- ^Bertram, L. (2009)."Genome-wide association studies in Alzheimer's disease".Human Molecular Genetics.18(R2): R137–R145.doi:10.1093/hmg/ddp406.PMC2758713.PMID19808789.
- ^Kellis, Manolis; Patterson, Nick; Endrizzi, Matthew; Birren, Bruce; Lander, Eric S. (15 May 2003). "Sequencing and comparison of yeast species to identify genes and regulatory elements".Nature.423(6937): 241–254.Bibcode:2003Natur.423..241K.doi:10.1038/nature01644.PMID12748633.S2CID1530261.
- ^Marchler-Bauer, A.; Lu, S.; Anderson, J. B.; Chitsaz, F.; Derbyshire, M. K.; DeWeese-Scott, C.; Fong, J. H.; Geer, L. Y.; Geer, R. C.; Gonzales, N. R.; Gwadz, M.; Hurwitz, D. I.; Jackson, J. D.; Ke, Z.; Lanczycki, C. J.; Lu, F.; Marchler, G. H.; Mullokandov, M.; Omelchenko, M. V.; Robertson, C. L.; Song, J. S.; Thanki, N.; Yamashita, R. A.; Zhang, D.; Zhang, N.; Zheng, C.; Bryant, S. H. (24 November 2010)."CDD: a Conserved Domain Database for the functional annotation of proteins".Nucleic Acids Research.39(Database): D225–D229.doi:10.1093/nar/gkq1189.PMC3013737.PMID21109532.
