Cyclotella
This article has multiple issues.Please helpimprove itor discuss these issues on thetalk page.(Learn how and when to remove these template messages)
|
| Cyclotella | |
|---|---|
| |
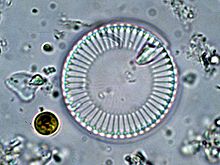
| Cyclotella meneghiniana | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Stramenopiles |
| Phylum: | Gyrista |
| Subphylum: | Ochrophytina |
| Class: | Bacillariophyceae |
| Order: | Thalassiosirales |
| Family: | Stephanodiscaceae |
| Genus: | Cyclotella (Kützing)de Brebisson |
Cyclotellais a genus ofdiatomsoften found inoligotrophicenvironments, both marine and fresh water. It is in the family Stephanodiscaceae and the orderThalassiosirales.[1]The genus was first discovered in the mid-1800s and since then has become an umbrella genus for nearly 100 different species, the most well-studied and the best known beingCyclotella meneghiniana.Despite being among the most dominant genera in low-productivity environments, it is relatively understudied.[2]
Cyclotella'shabitat has traditionally been described as low-productivitymesotrophicoroligotrophicfreshwater environments, but withC. meneghinianaappearing in warm, nutrient-rich environments as well as low-productivity environments, it has become unclear whether there is an archetypal aquatic setting for this genus.[3]
Etymology
[edit]The nameCyclotellais derived from theGreektermkyklos,meaning "circle." While "circle" can be used to describe many diatoms,Cyclotellaspp. are all circular and have a girdle band arrangement that makes the structure of the organism resemble a wheel.[4]
History
[edit]The genusCyclotellawas described in 1838 byLouis Alphonse de Brébisson,a French botanist and photographer.[5]Brébisson shares the credit of discovering the genus withFriedrich Traugott Kützing,a German pharmacist, botanist, and phycologist. This is in spite of the fact that neither one of these scientists ever worked together or even came in contact with one another. Kützing was a pioneer in microbial science, demonstrating the difference between diatoms anddesmidsin a German research paper in 1833. In 1849, Kützing published a comprehensive work describing 6000 differentalgaespecies, including the most known species ofCyclotellatoday-C. meneghiniana.[6]
As Brébisson describes in the 1838 publicationFlore de Normandie,Cyclotella"has a more or less elongated ovoid shape, it is swollen from both sides, and when its center is diaphanous, it resembles two tubular frustules united by their vertices ( translated from French )." Many databases, texts, and members of the scientific community refer to the entire genus of Cyclotella as Cyclotella (Kützing) Brébisson.[6]This full genus title indicates that Kützing initially discovered species of ageneraand put them into another genus, which was then altered by Brébisson who took some of those same species and placed them within the Cyclotella genus.[original research?]Upon distinguishingCyclotellafrom other diatom species, there have been nearly 100 different species of the genus described and taxonomically accepted.[citation needed]
Habitat and ecology
[edit]Species ofCyclotellaare most often found inoligotrophic(nutrient poor) environments. They are most often found in freshwater environments, but can also be found inbrackishand marine habitats as well. Many of the freshwater species have been found throughout the United States in stagnant waters.[7]Species that are most commonly found in marine environments areC. caspia,C. litoralis,C. meneghiniana,C. striata,andC. stylorwn.
In a study performed in 1974, it was determined that the optimalosmolarconcentration for growth inC. meneghinianain a medium of 0.5 Osm/L.[8]For references, the osmolarity of seawater is on average, 1 Osm/L.[4]Marine diatoms and algae in general tend to flourish in higherosmolarconcentrations due to the increased presence ofcarbon dioxideand nutrients to be utilized as sustenance, but the low-solute environment Schobert found to be most optimal for the growth ofC. meneghianais consistent with mostCyclotellabeing found in low-productivity mesotrophic to oligotrophic environments. Species of cyclotella have been found in harsh aquatic environments such as coldwater regions in northern regions of the world.[7]
Another study by Van de Vijver and Dessein found a new species ofCyclotella,C. deceusteriana,in thesub-antarcticregion.[9]One of the only ecological characteristics of Cyclotella that is consistent among most of its species is the fact that they are found in stagnant or near-stagnant waters and are immobile. Beyond that, there is a great deal of variation. Many of theCyclotellaspecies that have been studied have been shown to be found in aquatic environments that are either slightly or highlyalkaline.C. distinguendais known to prefer alkaline waters, and C. gamma has been found in lakes that have a pH range of 7.2 to 7.8. Nutrient concentration in the habitats ofCyclotellaspp. varies.C. sensulatohas been described as a dominant member of both mesotrophic and oligotrophic environments,[2]as many are, but both C. atomus and C. meneghiniana are found to prefer nutrient-rich environments. Temperature ranges vary between species as well; it was mentioned earlier thatC. deceusterianawas discovered in sub-antarctic regions, and C. gamma and C.quillensis have been found in the Northern United States andSaskatchewan,respectively.C. atomus,on the other hand, has been found in warmer lake sediments in California.Colonizationpatterns ofCyclotellaspp. are relatively uniform, in the sense that most of them are solitary organisms.C. meneghiniana,however, has been described to occasionally live in colonies.[10]Of course, the preference of nutrient rich environments ofC. meneghinianaconflicts the findings mentioned earlier.
Morphology
[edit]The size ofCyclotellavaries by species.C. atomushas a diameter of 5-7 μm, whereas C. quillensis can have a diameter up to 24-54 μm.[11]The most studied species of the genus, C. meneghiniana, has a diameter of 6-18 μm. Like all other diatoms,Cyclotellaspp. have transparent cell walls. They form biosilica shells using dissolvedsiliconandcarbonacquired from various carbon partitioning pathways.
Other materialsCyclotellaspp. use for cell wall biosynthesis are semiconductormetal oxidesand extracellular fibers made ofchitin.The primaryallomorphof chitin that is found most often in diatoms is α-chitin, butCyclotellaandThalassiosiracontain the β-chitin allomorph. Poly N-acetylglucosaminechains are oriented in a parallel manner and contain intermolecularhydrogen bonds.
The bond chains and hydrogen bonds between molecules form aparacrystallinematrix of β-chitin. This matrix contains pores large enough for whateversolventis available in the aquatic ecosystem in whichCyclotellaspp. reside in to enter the matrix and swell the structure.[12]
Diatoms are unique in the sense that they have valves, created by the two halves of a diatom's test.Cyclotellaspp. are no exception, as they form the upper and lower portions of the wall. The girdle bands that support the valves are thin strips of silica and ultimately circumscribe the cell. Each valve has two central tubes traversing its surface, meeting in the middle at the central nodule. Themorphologyof theCyclotellacell wall and its valves are important traits that distinguish species from each other. Each species has tangentially undulated valves all throughout their cell wall, regardless of their length, width, and concentration.[13]Frustulescontain areolas, that is orifices that mediate the passage of nutrients andexudatesacross the cell wall for sustenance. The characteristics of these areolas are thought to cause differences in mechanical strength and metabolism among different cells.[14]
Like other monoraphid diatoms,Cyclotellafrustules can be characterized as heterovalvar. The cell wall and cell membrane are what are known to this point as what distinguishesCyclotellafrom other diatom genera. Thecytoplasmiccomponents are assumed to be similar to what other diatoms have. InC. meneghiniana,there are granules scattered and attached at thechromatophoreall throughout the cytoplasm. The genus isphotosyntheticlike all other diatoms, so all species contain one or manypyrenoidstraversed by athylakoidmembrane and achloroplastwithin theendoplasmic reticulum.
Dictyosomesare also present in the cytoplasm, being in close proximity to the nucleus and making up thegolgi complex.The nucleus has been found to change locations inC. meneghinianathroughout generations as a result of the cell diameter gradually decreasing.[15]
Life cycle
[edit]Cyclotella meneghinianasplits in half duringasexual reproduction.The halves are separated by the distinction between the two valves for each cell. Each of the two offspring that arise as a result of cell division have one of the two valves from the parent cell. During the separation of the parent cell, the cytoplasm forms the two offspring valves that will end up complementing the inherited parent valves in the offspring once reproduction is complete.
The offspring valves are formed within a silica deposition vesicle that gradually grows larger and separates into two different offspring valves. The parent valves become a template for the offspring valves being formed, with patterns of striae and the central cell area also being inherited. However, perfectcomplementationdoes not occur every generation, which can lead to consecutive generations inheriting a deformed parental valve that was initially a deformed offspring valve in a previous generation. The likeness of the offspring valves to the parental valves is determined by the flexibility of the girdle bands; the other factors are unknown.[14]Vegetative cell division occurs over hundreds of generations forC. meneghiniana,with the cell diameters of the offspring organisms becoming gradually smaller. Regardless of the flexibility of the girdle bands and functionality ofvegetative celldivision, there is a point where the diameter ofC. meneghinianaoffspring dips below a certain threshold diameter. It has been observed that at this point, species-specific environmental stimuli induces the change from asexual reproductionto sexual reproduction.
Sexual reproduction occurs withgametesbeing formed upon reaching the threshold. During the process of meiosis, maleCyclotellacells releasespermand the femaleCyclotellacells develop and egg from within the two valves. Following fertilization of the egg, azygoteis formed from the union of the two gametes. The zygote then develops into an auxophore (2n). Once sexual reproduction is complete, the diameter of the offspring is larger and beyond the threshold once again, allowing for the production of another few hundred generations through the asexual division of auxophores.
Biochemistry
[edit]Despite there being very little known about the internal morphology ofCyclotella,there have been a sizable number of studies done on the genus'molecular biologyand genome.C. crypticahas been identified to be an oleaginous diatom, with a great deal of triacylglycerol. Itsgenomehas been identified to contain manymethylatedrepetitive sequences, which are supposed to function as a way of limiting the occurrences ofDNA transposition.C. crypticawas discovered to have a very efficient lipid metabolism, which is needed for its high triacylglycerol production.[16]
Another study conducted in 1992 indicates thatC. meninghianahas the largest genome and abundance of sequence repeats of any diatom species up to this specific study.[17]TheC. meninghianachloroplast genome alone has a vast amount ofequimolarinversion isomers. Many of these isomers differ in their orientation to their single copy sequence counterparts. The species, according to the findings, still has someprokaryoticand land plant gene clusters as well asoperons.In comparison to many other diatoms and plant chloroplast studies,C. meninghianahas a diversely rearranged gene order for single copy regions in its genome.[citation needed]
Fossil records
[edit]This section mayrequirecleanupto meet Wikipedia'squality standards.The specific problem is:References 20 and 21 were not found during cleanup. Such citations need to be found and added.(May 2020) |
FossilsofCyclotellaare not commonly discovered, however there have been a few species found fossilized in freshwater ecosystems. Fossil assemblages have been found in glacial and interglacial segments. Regardingtrophic levels,they have been found inoligotrophicand mesotrophic rivers in Europe andMediterranean regions.The frequent discovery ofC. distinguendafossils led to a consensus that they generally have an undulated central area.
A sample ofC. distinguendawas found at theAgios Florosfen, in SouthwestPeloponnese,Greece. The fossilized sample was dated to 5700 to 5300 years ago. Support for the recognition of a new diatom species,C. paradistinguenda,was proposed after looking through the sample ofC. distinguenda(20).C. paradistinguendawas dated back to 4600 years ago. Distinctions between the two species can also be described in the differences instratigraphicdistributions between the two, asC. paradistinguendawas found in an upper organic sequence of the sample compared toC. distinguenda(20).
Another sample of Cyclotella was found atLake Petén-Itzá,lowlandGuatemala.The newfound diatom species were found fossilized morphologically distinct from otherCyclotellaspecies (21). One of the species was namedC. petenensis.The other species was namedC. cassandrae,characterized by its elliptically shaped valve paired with its coarse striae. Most notably it has a scattered ring of central fultoportulae (21). Discovering fossils is not often a credible enough way to determine a new species within the phylum of diatoms, given that determining underlying mechanisms based on morphological variability is unreliable. It's best to use both morphological and paleoecological data obtained from samples- the two are often difficult to obtain just from fossils (20).
References
[edit]- ^Kociolek, J.P.; Balasubramanian, K.; Blanco, S.; Coste, M.; Ector, L.; Liu, Y.; Kulikovskiy, M.; Lundholm, N.; Ludwig, T.; Potapova, M.; Rimet, F.; Sabbe, K.; Sala, S.; Sar, E.; Taylor, J.; Van de Vijver, B.; Wetzel, C.E.; Williams, D.M.; Witkowski, A.; Witkowski, J. (2020)."Cyclotella (F.T. Kützing) A. de Brébisson, 1838".WoRMS.World Register of Marine Species.Retrieved11 May2020.
- ^abSaros, J.E., Anderson, N.J. (2015). The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol Rev Camb Philos Soc. 90(2). 522-41.
- ^Tanaka, Hiroyuki (2007).Taxonomic Studies of the Genera Cyclotella (Kützing) Brébisson, Discostella Houk Et Klee, and Puncticulata Håkansson in the Family Sephanodiscaceae Glezer Et Makarova (Bacillariophyta) in Japan.J. Cramer.ISBN978-3-443-57044-6.
- ^abBrébisson, [L.] A. de (1838). Considérations sur les diatomées et essai d'une classification des genres et des espèces appartenant à cette famille, par A. de Brébisson, auteur de la Flore de Normandie, etc. pp. [i], [1]-20, [4, err.]. Falaise & Paris: Brée l'Ainée Imprimeur-Libraire; Meilhac.
- ^Håkansson H. (2002). A compilation and evaluation of species in the genera Stephanodiscus, Cyclostephanos and Cyclotella with a new genus in the family Stephanodiscaceae. Diatom Research. 17(1): 1-139.
- ^abKützing, F.T. (1844). Die kieselschaligen Bacillarien oder Diatomeen. Nordhausen. 30. 1-152
- ^abHasle, G.R., and E.E. Syvertsen. (1997). Marine Diatoms. In: Tomas, C.R. (Ed.) Identifying Marine Phytoplankton. Academic Press.
- ^Schobert, B. (1974). The influence of water stress on the metabolism of diatoms I. Osmotic resistance and proline accumulation in Cyclotella meneghiniana. Zeitschrift für Pflanzenphysiologie. 74(2). 106-120.
- ^Van de Vijver, Bart & Dessein, Steven. (2018). Cyclotella deceusteriana, a new centric diatom species (Bacillariophyta) from the sub-Antarctic Region. Phytotaxa. 333(1).
- ^Lowe, R.L. (1975). Comparative ultrastructure of the valves of some Cyclotella species (Bacillariophyceae) Journal of Phycology. 11(4): 415-424.
- ^Bailey, L.W. (1922). Diatoms from the Quill Lakes, Saskatchewan, and from Airdrie, Alberta.Contributions to Canadian Biology 11(1): 157-165.
- ^LeDuff, P., & Rorrer, G. L. (2019). Formation of extracellular β-chitin nanofibers during batch cultivation of marine diatom Cyclotella sp. at silicon limitation. Journal of Applied Phycology, 31(6), 3479–3490.
- ^Tesson, B., Hildebrand, M. (2010). Dynamics of silica cell wall morphogenesis in the diatom Cyclotella cryptica: Substructure formation and the role of microfilaments. Journal of Structural Biology. 169(1). 62-74.
- ^abShirokawa, Y., Shimada, M. (2016). Cytoplasmic inheritance of parent–offspring cell structure in the clonal diatom Cyclotella meneghiniana. Proceedings of the Royal Society B. 283(1842).
- ^Hoops, H.J., Floyd, G.L. (1979). Ultrastructure of the centric diatom, Cyclotella meneghiniana: vegetative cell and auxospore development. Phycologia. 18(4). 424-435.
- ^Traller, J.C., Cokus, S.J., Lopez, D.A. et al. (2016). Genome and methylome of the oleaginous diatom Cyclotella cryptica reveal genetic flexibility toward a high lipid phenotype. Biotechnol Biofuels. 9(258).
- ^Bourne, C.E.M. (1992). Chloroplast DNA structure, variation and phylogeny in closely related species of Cyclotella. ProQuest Dissertations Publishing.
- Crawford, B.J., Burke, R.D. (2004). Development of Sea Urchins, Ascidians, and Other Invertebrate Deuterostomes: Experimental Approaches. Methods in Cell Biology. 74(1). 411–441.
- Harvey, B.P., Agostini, S., Kon, K., Wada, S., Hall-Spencer, J.M. (2019). Diatoms Dominate and Alter Marine Food-Webs When CO2 Rises. Diversity. 11(12). 242.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The diatoms. Biology and morphology of the genera. Cambridge University Press, Cambridge.
- Spaulding, S., Edlund, M. (2008). Cyclotella. In Diatoms of North America. Retrieved April 2, 2020, fromhttps://diatoms.org/genera/cyclotella
