Dimethyl sulfoxide
| |||
| |||
 A sample of dimethyl sulfoxide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Methanesulfinyl)methane | |||
| Systematic IUPAC name
(Methanesulfinyl)methane(substitutive) Dimethyl(oxido)sulfur(additive) | |||
| Other names
Methylsulfinylmethane
Methyl sulfoxide (2:1), Dermasorb[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | DMSO, Me2SO | ||
| 506008 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.604 | ||
| EC Number |
| ||
| 1556 | |||
| KEGG | |||
| MeSH | Dimethyl+sulfoxide | ||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H6OS | |||
| Molar mass | 78.13g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 1.1004g⋅cm−3 | ||
| Melting point | 19 °C (66 °F; 292 K) | ||
| Boiling point | 189 °C (372 °F; 462 K) | ||
| Miscible | |||
| SolubilityinDiethyl ether | Not soluble | ||
| Vapor pressure | 0.556 millibars or 0.0556 kPa at 20 °C[2] | ||
| Acidity(pKa) | 35[3] | ||
Refractive index(nD)
|
1.479 εr= 48 | ||
| Viscosity | 1.996cPat 20 °C | ||
| Structure | |||
| Cs | |||
| Trigonal pyramidal | |||
| 3.96D | |||
| Pharmacology | |||
| G04BX13(WHO)M02AX03(WHO) | |||
| Hazards | |||
| Occupational safety and health(OHS/OSH): | |||
Main hazards
|
Irritant | ||
| NFPA 704(fire diamond) | |||
| Flash point | 89 °C (192 °F; 362 K) | ||
| Safety data sheet(SDS) | Oxford MSDS | ||
| Related compounds | |||
Relatedsulfoxides
|
Diethyl sulfoxide | ||
Related compounds
|
|||
| Supplementary data page | |||
| Dimethyl sulfoxide (data page) | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Dimethyl sulfoxide(DMSO) is anorganosulfur compoundwith theformula(CH3)2SO.This colorless liquid is thesulfoxidemost widely used commercially. It is an importantpolaraprotic solventthat dissolves bothpolar and nonpolarcompounds and ismisciblein a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO is metabolised to compounds that leave agarlic-like taste in the mouth after DMSO is absorbed by skin.[5]
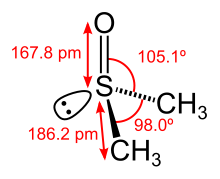
In terms of chemical structure, the molecule has idealizedCssymmetry.It has atrigonal pyramidal molecular geometryconsistent with other three-coordinate S(IV) compounds,[6]with anonbonded electron pairon the approximatelytetrahedralsulfur atom.
Synthesis and production
[edit]Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientistAlexander Zaytsev,who reported his findings in 1867.[7]Its modern use as an industrial solvent began through popularization by Thor Smedslund at the Stepan Chemical Company.[8]Dimethyl sulfoxide is produced industrially fromdimethyl sulfide,a by-product of theKraft process,by oxidation with oxygen ornitrogen dioxide.[9]
Reactions
[edit]Reactions with electrophiles
[edit]The sulfur center in DMSO isnucleophilictoward softelectrophilesand the oxygen is nucleophilic toward hard electrophiles. Withmethyl iodideit formstrimethylsulfoxonium iodide,[(CH3)3SO]I:
- (CH3)2SO + CH3I → [(CH3)3SO]I
This salt can bedeprotonatedwithsodium hydrideto form thesulfurylide:
- [(CH3)3SO]I + NaH → (CH3)2S(CH2)O + NaI + H2
Acidity
[edit]The methyl groups of DMSO are only weakly acidic, with apKa= 35.For this reason, the basicities of many weakly basic organic compounds have been examined in this solvent.
Deprotonation of DMSO requires strong bases likelithium diisopropylamideandsodium hydride.Stabilization of the resultantcarbanionis provided by the S(O)R group. The sodium derivative of DMSO formed in this way is referred to asdimsyl sodium.It is a base, e.g., for the deprotonation ofketonesto form sodiumenolates,phosphonium saltsto formWittig reagents,andformamidiniumsalts to formdiaminocarbenes.It is also a potent nucleophile.
Oxidant
[edit]Inorganic synthesis,DMSO is used as a mild oxidant.[10]It forms the basis of several selectivesulfonium-based oxidation reactionsincluding thePfitzner–Moffatt oxidation,Corey–Kim oxidationand theSwern oxidation.[11]TheKornblum oxidationis conceptually similar. These all involve formation of an intermediatesulfoniumspecies (R2S+X where X is a heteroatom)
Ligand and Lewis base
[edit]Related to its ability to dissolve many salts, DMSO is a commonligandincoordination chemistry.[12]Illustrative is the complexdichlorotetrakis(dimethyl sulfoxide)ruthenium(II)(RuCl2(dmso)4). In this complex, three DMSO ligands are bonded torutheniumthrough sulfur. The fourth DMSO is bonded through oxygen. In general, the oxygen-bonded mode is more common.
In carbon tetrachloride solutions DMSO functions as a Lewis base with a variety of Lewis acids such asI2,phenols,trimethyltin chloride,metalloporphyrins, and the dimerRh2Cl2(CO)4.The donor properties are discussed in theECW model.The relative donor strength of DMSO toward a series of acids, versus other Lewis bases, can be illustrated byC-B plots.[13][14]
Applications
[edit]Solvent
[edit]
DMSO is apolar aprotic solventand is less toxic than other members of this class, such asdimethylformamide,dimethylacetamide,N-methyl-2-pyrrolidone,andhexamethylphosphoramide(HMPA). DMSO is frequently used as asolventfor chemical reactions involving salts, most notablyFinkelstein reactionsand othernucleophilic substitutions.It is also extensively used as an extractant in biochemistry and cell biology.[15]Because DMSO is only weakly acidic, it tolerates relatively strong bases and as such has been extensively used in the study ofcarbanions.A set of non-aqueouspKavalues (C-H, O-H, S-H and N-H acidities) for thousands of organic compounds have been determined in DMSO solution.[16][17]
Because of its high boiling point, 189 °C (372 °F), DMSO evaporates slowly at normal atmospheric pressure. Samples dissolved in DMSO cannot as easily be recovered compared to other solvents, as it is very difficult to remove all traces of DMSO by conventionalrotary evaporation.One technique to fully recover samples is removal of the organic solvent by evaporation followed by addition of water (to dissolve DMSO) andcryodesiccationto remove both DMSO and water. Reactions conducted in DMSO are often diluted with water to precipitate or phase-separate products. The relatively high freezing point of DMSO, 18.5 °C (65.3 °F), means that at, or just below, room temperature it is a solid, which can limit its utility in some chemical processes (e.g.crystallizationwith cooling).
In itsdeuteratedform (DMSO-d6), it is a useful solvent forNMRspectroscopy, again due to its ability to dissolve a wide range of analytes, the simplicity of its own spectrum, and its suitability for high-temperature NMR spectroscopic studies. Disadvantages to the use of DMSO-d6are its high viscosity, which broadens signals, and itshygroscopicity,which leads to an overwhelming H2O resonance in the1H-NMR spectrum. It is often mixed withCDCl3orCD2Cl2for lower viscosity and melting points.
DMSO is used to dissolve test compounds inin vitrodrug discovery[18][19]anddrug design[20]screeningprograms, includinghigh-throughput screeningprograms.[19][20]This is because it is able to dissolve bothpolarandnonpolarcompounds,[18][20]can be used to maintainstock solutionsof test compounds (important when working with a largechemical library),[19]is readilymisciblewith water andcell culture media,and has a high boiling point (this improves the accuracy of test compound concentrations by reducing room temperature evaporation).[18]One limitation with DMSO is that it can affectcell linegrowth and viability, with low DMSO concentrations sometimes stimulating cell growth, and high DMSO concentrations sometimes inhibiting or killing cells.[18]
DMSO is used as a vehicle inin vivostudies of test compounds. It has, for example, been employed as a co-solvent to assist absorption of theflavonol glycosideIcariinin thenematodewormCaenorhabditis elegans.[21]As with its use inin vitrostudies, DMSO has some limitations inanimal models.[22][23]Pleiotropiceffects can occur and, if DMSO control groups are not carefully planned, then solvent effects can falsely be attributed to the prospective drug.[22]For example, even a very low dose of DMSO has a powerful protective effect againstparacetamol(acetaminophen)-induced liver injury in mice.[23]
DMSO is finding increased use in manufacturing processes to produce microelectronic devices.[24]It is widely used to strip photoresist in TFT-LCD 'flat panel' displays and advanced packaging applications (such as wafer-level packaging / solder bump patterning). DMSO is an effectivepaint stripper,being safer than many of the others such asnitromethaneanddichloromethane.
Biology
[edit]DMSO is used inpolymerase chain reaction(PCR) to inhibitsecondary structuresin theDNA templateor theDNA primers.It is added to the PCR mix before reacting, where it interferes with the self-complementarity of the DNA, minimizing interfering reactions.[25]
DMSO in a PCR is applicable for supercoiled plasmids (to relax before amplification) or DNA templates with highGC-content(to decreasethermostability). For example, 10% final concentration of DMSO in the PCR mixture with Phusion decreases primer annealing temperature (i.e. primer melting temperature) by 5.5–6.0 °C (9.9–10.8 °F).[26]
It is well known as a reversible cell cycle arrester at phase G1 of human lymphoid cells.[27]
DMSO may also be used as acryoprotectant,added to cell media to reduce ice formation and thereby prevent cell death during the freezing process.[28]Approximately 10% may be used with a slow-freeze method, and the cells may be frozen at −80 °C (−112 °F) or stored inliquid nitrogensafely.
In cell culture, DMSO is used to inducedifferentiationofP19 embryonic carcinoma cellsintocardiomyocytesandskeletal muscle cells.
Medicine
[edit]Use of DMSO in medicine dates from around 1963, when anOregon Health & Science UniversityMedical School team, headed byStanley Jacob,discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system. In medicine, DMSO is predominantly used as a topicalanalgesic,a vehicle for topical application of pharmaceuticals, as ananti-inflammatory,and anantioxidant.[29]Because DMSO increases the rate of absorption of some compounds throughbiological tissues,includingskin,it is used in sometransdermaldrug deliverysystems. Its effect may be enhanced with the addition ofEDTA.It is frequently compounded with antifungal medications, enabling them to penetrate not just skin but also toenails and fingernails.[30]
DMSO has been examined for the treatment of numerous conditions and ailments, but the U.S.Food and Drug Administration(FDA) has approved its use only for the symptomatic relief of patients withinterstitial cystitis.[31]A 1978 study concluded that DMSO broughtsignificantrelief to the majority of the 213 patients with inflammatorygenitourinarydisorders that were studied.[32]The authors recommended DMSO for genitourinary inflammatory conditions not caused by infection or tumor in which symptoms were severe or patients failed to respond to conventional therapy.
Ininterventional radiology,DMSO is used as a solvent forethylene vinyl alcoholin theOnyxliquid embolic agent, which is used inembolization,the therapeutic occlusion of blood vessels.
IncryobiologyDMSO has been used as acryoprotectantand is still an important constituent of cryoprotectantvitrificationmixtures used to preserve organs, tissues, and cell suspensions. Without it, up to 90% of frozen cells will become inactive. It is particularly important in the freezing and long-term storage ofembryonic stem cellsandhematopoietic stem cells,which are often frozen in a mixture of 10% DMSO, a freezing medium, and 30%fetal bovine serum.In the cryogenic freezing of heteroploid cell lines (MDCK,VERO,etc.) a mixture of 10% DMSO with 90%EMEM(70% EMEM + 30% fetal bovine serum + antibiotic mixture) is used. As part of anautologousbone marrow transplantthe DMSO is re-infused along with the patient's ownhematopoietic stem cells.
DMSO is metabolized bydisproportionationtodimethyl sulfideanddimethyl sulfone.It is subject to renal and pulmonary excretion. A possible side effect of DMSO is therefore elevated blood dimethyl sulfide, which may cause a blood bornehalitosissymptom.
Alternative medicine
[edit]DMSO is marketed as analternative medicine.Its popularity as an alternative cure is stated to stem from a60 Minutesdocumentary in 1980 featuring an early proponent.[33]However, DMSO is an ingredient in some products listed by the U.S. FDA as fake cancer cures[34]and the FDA has had a running battle with distributors.[33]One such distributor is Mildred Miller, who promoted DMSO for a variety of disorders and was consequently convicted ofMedicare fraud.[33]
The use of DMSO as an alternative treatment for cancer is of particular concern, as it has been shown to interfere with a variety ofchemotherapydrugs, includingcisplatin,carboplatin,andoxaliplatin.[35]There is insufficient evidence to support the hypothesis that DMSO has any effect,[36]and most sources agree that its history of side effects when tested warrants caution when using it as a dietary supplement, for which it is marketed heavily with theusual disclaimer.
Veterinary medicine
[edit]DMSO is commonly used in veterinary medicine as alinimentforhorses,alone or in combination with other ingredients. In the latter case, often, the intended function of the DMSO is as a solvent, to carry the other ingredients across the skin. Also in horses, DMSO is used intravenously, again alone or in combination with other drugs. It is used alone for the treatment of increased intracranial pressure and/or cerebral edema in horses.[citation needed]
Taste
[edit]The perceived garlic taste upon skin contact with DMSO may be due tononolfactoryactivation ofTRPA1receptors intrigeminal ganglia.[37]Unlikedimethylanddiallyldisulfides (which have odors resembling garlic),mono-andtri-sulfides (which typically have foul odors), and similar odiferous sulfur compounds, the pure chemical DMSO is odorless.
Safety
[edit]Toxicity
[edit]DMSO is a non-toxic solvent with amedian lethal dosehigher than ethanol (DMSO: LD50,oral, rat, 14,500 mg/kg;[38][39]ethanol: LD50,oral, rat, 7,060 mg/kg[40]).
DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects. DMSO is thought to increase the effects of blood thinners, steroids, heart medicines, sedatives, and other drugs. In some cases this could be harmful or dangerous.[41]
Because DMSO easily penetrates theskin,substances dissolved in DMSO may quickly be absorbed.Gloveselection is important when working with DMSO.Butyl rubber,fluoroelastomer,neoprene,or thick (15mil/ 0.4mm)latexgloves are recommended.[42]Nitrilegloves, which are very commonly used in chemical laboratories, may protect from brief contact but have been found to degrade rapidly with exposure to DMSO.[43]
Regulation
[edit]In Australia, it is listed as aSchedule 4 (S4)Drug, and a company has been prosecuted for adding it to products as a preservative.[44]
Clinical safety
[edit]Early clinical trials with DMSO were stopped because of questions about its safety, especially its ability to harm the eye. The most commonly reported side effects include headaches and burning and itching on contact with the skin. Strong allergic reactions have been reported.[full citation needed]
On September 9, 1965,The Wall Street Journalreported that a manufacturer of the chemical warned that the death of an Irish woman after undergoing DMSO treatment for a sprained wrist may have been due to the treatment, although no autopsy was done, nor was a causal relationship established.[45]Clinical researchusing DMSO was halted and did not begin again until theNational Academy of Sciences(NAS) published findings in favor of DMSO in 1972.[46]In 1978, the US FDA approved DMSO for treatinginterstitial cystitis.In 1980, the US Congress held hearings on claims that the FDA was slow in approving DMSO for other medical uses. In 2007, the US FDA granted "fast track" designation on clinical studies of DMSO's use in reducing brain tissue swelling followingtraumatic brain injury.[46]
DMSO exposure to developing mouse brains can produce brain degeneration. Thisneurotoxicitycould be detected atdosesas low as 0.3mL/kg, a level exceeded in children exposed to DMSO duringbone marrow transplant.[47]
Odor problem
[edit]DMSO disposed intosewerscan cause odor problems in municipal effluents: waste waterbacteriatransform DMSO underhypoxic(anoxic) conditions intodimethyl sulfide(DMS) that has a strong disagreeable odor, similar to rotten cabbage.[48]However, chemically pure DMSO is odorless because of the lack of C-S-C (sulfide) and C-S-H (mercaptan) linkages. Deodorization of DMSO is achieved by removing the odorous impurities it contains.[49]
Explosion hazard
[edit]Dimethyl sulfoxide can produce an explosive reaction when exposed toacyl chlorides;at a low temperature, this reaction produces theoxidantforSwern oxidation.
DMSO can decompose at the boiling temperature of 189 °C at normal pressure, possibly leading to an explosion. The decomposition is catalyzed by acids and bases and therefore can be relevant at even lower temperatures. A strong to explosive reaction also takes place in combination with halogen compounds, metal nitrides, metal perchlorates, sodium hydride, periodic acid and fluorinating agents.[50]
See also
[edit]- Varying oxidation of sulfur
- Dimethyl sulfide(DMS), the corresponding sulfide, also produced by marine phytoplankton and emitted to the oceanic atmosphere where it is oxidized to DMSO, SO2and sulfate
- Dimethyl sulfone, commonly known asmethylsulfonylmethane(MSM), a related chemical often marketed as a dietary supplement
- Related compounds with methyl on oxygen
- Dimethyl sulfite,the corresponding sulfite
- Dimethyl sulfate(also DMS), the corresponding sulfate: amutagenicalkylatingcompound
- Methyl methanesulfonate,another methylating agent
- Gloria Ramirez,also known as the "Toxic Woman"
References
[edit]- ^DMSO (medication)
- ^"Dimethyl Sulfoxide (DMSO) -- Technical".Atofina Chemicals, inc.Retrieved26 May2007.
- ^Matthews WS, Bares JE, Bartmess JE, Bordwell FG, Cornforth FJ, Drucker GE, Margolin Z, McCallum RJ, McCollum GJ, Vanier NR (1975). "Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution".J. Am. Chem. Soc.97(24): 7006–7014.doi:10.1021/ja00857a010.
- ^"Dimethyl sulfoxide".pubchem.ncbi.nlm.nih.gov.
- ^Novak KM, ed. (2002).Drug Facts and Comparisons(56th ed.). St. Louis, Missouri: Wolters Kluwer Health. p.2345.ISBN978-1-57439-110-7.
- ^Thomas R, Shoemaker CB, Eriks K (1966). "The Molecular and Crystal Structure of Dimethyl Sulfoxide, (H3C)2SO ".Acta Crystallogr.21(1): 12–20.Bibcode:1966AcCry..21...12T.doi:10.1107/S0365110X66002263.
- ^von Demselben (1867). "Ueber die Einwirkung von Saltpetersäure auf Schwefelmethyl und Schwefeläthyl" [On the effect of nitric acid on methyl sulfide and ethyl sulfide]. In Erlenmeyer, E.; Rieckher, T.; Volhard, J.; Liebig, J.; Wöhler, F. (eds.).Annalen der Pharmacie(in German). Meyer; Winter. p. 148.
- ^Gergel, Max G.(March 1977).Excuse me sir, would you like to buy a kilo of isopropyl bromide?.Pierce Chemical. p. 145.
- ^Roy, Kathrin-Maria (15 June 2000), "Sulfones and Sulfoxides",Ullmann's Encyclopedia of Industrial Chemistry,Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA,doi:10.1002/14356007.a25_487,ISBN3527306730
- ^Epstein WW, Sweat FW (March 1967). "Dimethyl Sulfoxide Oxidations".Chemical Reviews.67(3): 247–260.doi:10.1021/cr60247a001.PMID6042131.
- ^Tidwell TT (1990). "Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update".Synthesis.1990(10): 857–870.doi:10.1055/s-1990-27036.
- ^Calligaris M (2004). "Structure and bonding in metal sulfoxide complexes: An update".Coordination Chemistry Reviews.248(3–4): 351–375.doi:10.1016/j.ccr.2004.02.005.
- ^Laurence, Christian; Gal, Jean-François (2010).Lewis basicity and affinity scales: data and measurement.Chichester, West Sussex, U.K.: John Wiley. pp. 50–51.ISBN978-0-470-74957-9.OCLC428031803.
- ^Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases".Journal of Chemical Education.54:612–613.doi:10.1021/ed054p612.The plots shown in this paper used older parameters. Improved E&C parameters are listed inECW model.
- ^"DMSO".exactantigen.Archived fromthe originalon 2009-10-05.Retrieved2009-10-02.
- ^ Bordwell FG (1988). "Equilibrium acidities in dimethyl sulfoxide solution".Accounts of Chemical Research.21(12): 456–463.doi:10.1021/ar00156a004.S2CID26624076.
- ^ "Bordwell pKa Table (Acidity in DMSO)".Archivedfrom the original on 9 October 2008.Retrieved23 April2019.
- ^abcdCushnie TP, Cushnie B, Echeverría J, Fowsantear W, Thammawat S, Dodgson JL, Law S, Clow SM (June 2020)."Bioprospecting for antibacterial drugs: a multidisciplinary perspective on natural product source material, bioassay selection and avoidable pitfalls".Pharmaceutical Research.37(7): Article 125.doi:10.1007/s11095-020-02849-1.PMID32529587.S2CID219590658.
- ^abcIlouga PE, Winkler D, Kirchhoff C, Schierholz B, Wölcke J (November 2007)."Investigation of 3 industry-wide applied storage conditions for compound libraries".Journal of Biomolecular Screening.12(1): 21–32.doi:10.1177/1087057106295507.PMID17099243.
- ^abcBalakin KV, Savchuk NP, Tetko IV (2006). "In silico approaches to prediction of aqueous and DMSO solubility of drug-like compounds: trends, problems and solutions".Current Medicinal Chemistry.13(2): 223–241.doi:10.2174/092986706775197917.PMID16472214.
- ^Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY (2011). Blagosklonny MV (ed.)."Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans".PLOS ONE.6(12): e28835.Bibcode:2011PLoSO...628835C.doi:10.1371/journal.pone.0028835.PMC3244416.PMID22216122.
- ^abKelava T, Cavar I (Nov 2011)."Biological actions of drug solvents".Periodicum Biologorum.113(3): 311–320.
- ^abKelava T, Cavar I, Čulo F (Oct 2010). "Influence of small doses of various drug vehicles on acetaminophen-induced liver injury".Can J Physiol Pharmacol.88(10): 980–87.doi:10.1139/Y10-065.PMID20962895.
- ^Kvakovszky G, McKim AS, Moore J (2007). "A Review of Microelectronic Manufacturing Applications Using DMSO-Based Chemistries".ECS Transactions.11(2): 227–234.Bibcode:2007ECSTr..11b.227K.doi:10.1149/1.2779383.S2CID137979405.
- ^ Chakrabarti R, Schutt CE (August 2001). "The enhancement of PCR amplification by low molecular-weight sulfones".Gene.274(1–2): 293–298.doi:10.1016/S0378-1119(01)00621-7.PMID11675022.
- ^"Guidelines for PCR Optimization with Phusion High-Fidelity DNA Polymerase".
- ^ Sawai M, Takase K, Teraoka H, Tsukada K (1990). "Reversible G1 arrest in the cell cycle of human lymphoid cell lines by dimethyl sulfoxide".Exp. Cell Res.187(1): 4–10.doi:10.1016/0014-4827(90)90108-m.PMID2298260.
- ^
Pegg, DE (2007). "Principles of Cryopreservation". In Day JG, Stacey GN (eds.).Cryopreservation and Freeze-Drying Protocols.Methods in Molecular Biology. Vol. 368. Humana Press. pp. 39–57.doi:10.1007/978-1-59745-362-2_3.ISBN978-1-58829-377-0.ISSN1064-3745.PMID18080461.
{{cite book}}:|journal=ignored (help) - ^Johannes Geiss (2001).The century of space science.Kluwer Academic. p. 20.ISBN978-0-7923-7195-3.Retrieved2011-08-07.
- ^Capriotti K, Capriotti JA (2015-10-08)."Onychomycosis treated with a dilute povidone-iodine/dimethyl sulfoxide preparation".International Medical Case Reports Journal.8:231–233.doi:10.2147/IMCRJ.S90775.PMC4599634.PMID26491374.
- ^"Import Alert 62-06".accessdata.fda.gov.Archived fromthe originalon 2017-02-04.Retrieved2017-03-05.
- ^ Shirley SW, Stewart BH, Mirelman S (March 1978)."Dimethyl Sulfoxide in Treatment of Inflammatory Genitourinary Disorders".Urology.11(3): 215–220.doi:10.1016/0090-4295(78)90118-8.PMID636125.
- ^abcJarvis WT (24 November 2001)."DMSO".National Council Against Health Fraud.Retrieved19 July2022.
- ^"187 Fake Cancer" Cures "Consumers Should Avoid".FDA.Archived fromthe originalon 23 July 2017.
- ^Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, et al. (July 2014)."Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes".Cancer Research.74(14): 3913–3922.doi:10.1158/0008-5472.CAN-14-0247.PMC4153432.PMID24812268.
- ^Saling, Joseph (20 June 2022)."DMSO: Uses and Risks".WebMD.Retrieved19 July2022.
- ^Lübbert M, Kyereme J, Schöbel N, Beltrán L, Wetzel CH, Hatt H (October 21, 2013)."Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons".PLOS ONE.8(10): e77998.Bibcode:2013PLoSO...877998L.doi:10.1371/journal.pone.0077998.PMC3804614.PMID24205061.
- ^"Safety Data Sheet: Dimethyl Sulfoxide (DMSO)"(PDF).Gaylord Chemical Company, L.L.C. 21 July 2016.Archived(PDF)from the original on 13 February 2019.
- ^"Material Safety Data Sheet: Dimethyl Sulfoxide".ScienceLab.21 May 2013. Archived fromthe originalon 19 September 2018.
- ^"Material Safety Data Sheet: Ethyl alcohol 200 Proof".ScienceLab.21 May 2013. Archived fromthe originalon 19 September 2018.
- ^"DMSO".American Cancer Society.Archived fromthe originalon 27 July 2010.
- ^Rubber Chemical Resistance Chart
- ^"Chemical hygiene plan"(PDF).Cornell University.October 1999.Retrieved2010-04-12.
- ^"Brisbane drug company convicted of counterfeiting".Commonwealth of Australia: Department of Health and Ageing. 23 April 2003. Archived fromthe originalon 2012-03-21.
- ^Carley W (September 9, 1965). "DMSO may have caused death of woman, makers of 'Wonder' drug warn doctors".The Wall Street Journal.New York City.
- ^abhttps:// fda.gov/ForIndustry/ImportProgram/ImportAlerts/ucm162294.htm[dead link]
- ^Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB (April 2009)."Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system".Neurobiology of Disease.34(1): 1–10.doi:10.1016/j.nbd.2008.11.006.PMC2682536.PMID19100327.
- ^Glindemann D, Novak J, Witherspoon J (January 2006). "Dimethyl sulfoxide (DMSO) waste residues and municipal waste water odor by dimethyl sulfide (DMS): the North-East WPCP plant of Philadelphia".Environmental Science and Technology.40(1): 202–207.Bibcode:2006EnST...40..202G.doi:10.1021/es051312a.PMID16433352.
- ^US application 2009005601A1,George Kvakovszky; David Villarrubia II & Scott Stevenson et al., "Process for preparing low malodorous dimethyl sulfoxide", published 2009, assigned to Gaylord Chemical Company LLC
- ^Roth, Lutz; Weller, Ursula (August 2000).Gefährliche Chemische Reaktionen[Dangerous Chemical Reactions]. Ecomed Sicherheit (in German). Landsberg/Lech: Verlagsgruppe Hüthig Jehle Rehm.ISBN3-609-73090-0.CD-ROM:ISBN3-609-48040-8





