DNA
| Part of a series on |
| Genetics |
|---|
 |
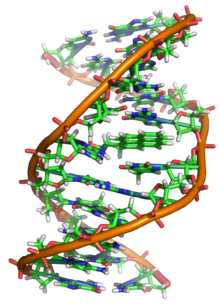
Deoxyribonucleic acid(/diːˈɒksɪˌraɪboʊnjuːˌkliːɪk,-ˌkleɪ-/;[1]DNA) is apolymercomposed of twopolynucleotidechains that coil around each other to form adouble helix.The polymer carriesgeneticinstructions for the development, functioning, growth andreproductionof all knownorganismsand manyviruses.DNA andribonucleic acid(RNA) arenucleic acids.Alongsideproteins,lipidsand complex carbohydrates (polysaccharides), nucleic acids are one of the four major types ofmacromoleculesthat are essential for all known forms oflife.
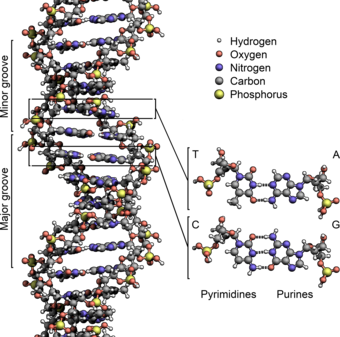
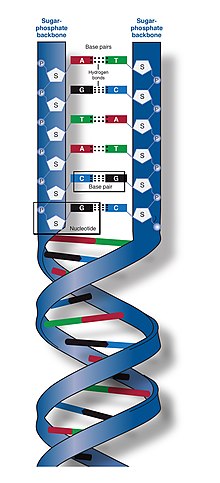
The two DNA strands are known as polynucleotides as they are composed of simplermonomericunits callednucleotides.[2][3]Each nucleotide is composed of one of fournitrogen-containingnucleobases(cytosine[C],guanine[G],adenine[A] orthymine[T]), asugarcalleddeoxyribose,and aphosphate group.The nucleotides are joined to one another in a chain bycovalent bonds(known as thephosphodiester linkage) between the sugar of one nucleotide and the phosphate of the next, resulting in an alternatingsugar-phosphate backbone.The nitrogenous bases of the two separate polynucleotide strands are bound together, according tobase pairingrules (A with T and C with G), withhydrogen bondsto make double-stranded DNA. The complementary nitrogenous bases are divided into two groups, the single-ringedpyrimidinesand the double-ringedpurines.In DNA, the pyrimidines are thymine and cytosine; the purines are adenine and guanine.
Both strands of double-stranded DNA store the samebiological information.This information isreplicatedwhen the two strands separate. A large part of DNA (more than 98% for humans) isnon-coding,meaning that these sections do not serve as patterns forprotein sequences.The two strands of DNA run in opposite directions to each other and are thusantiparallel.Attached to each sugar is one of four types of nucleobases (orbases). It is thesequenceof these four nucleobases along the backbone that encodes genetic information.RNAstrands are created using DNA strands as a template in a process calledtranscription,where DNA bases are exchanged for their corresponding bases except in the case of thymine (T), for which RNA substitutesuracil(U).[4]Under thegenetic code,these RNA strands specify the sequence ofamino acidswithin proteins in a process calledtranslation.
Within eukaryotic cells, DNA is organized into long structures calledchromosomes.Before typicalcell division,these chromosomes are duplicated in the process of DNA replication, providing a complete set of chromosomes for each daughter cell.Eukaryotic organisms(animals,plants,fungiandprotists) store most of their DNA inside thecell nucleusasnuclear DNA,and some in themitochondriaasmitochondrial DNAor inchloroplastsaschloroplast DNA.[5]In contrast,prokaryotes(bacteriaandarchaea) store their DNA only in thecytoplasm,incircular chromosomes.Within eukaryotic chromosomes,chromatinproteins, such ashistones,compact and organize DNA. These compacting structures guide the interactions between DNA and other proteins, helping control which parts of the DNA are transcribed.
Properties
DNA is a longpolymermade from repeating units callednucleotides.[6][7]The structure of DNA is dynamic along its length, being capable of coiling into tight loops and other shapes.[8]In all species it is composed of two helical chains, bound to each other byhydrogen bonds.Both chains are coiled around the same axis, and have the samepitchof 34ångströms(3.4nm). The pair of chains have a radius of 10 Å (1.0 nm).[9]According to another study, when measured in a different solution, the DNA chain measured 22–26 Å (2.2–2.6 nm) wide, and one nucleotide unit measured 3.3 Å (0.33 nm) long.[10]The buoyant density of most DNA is 1.7g/cm3.[11]
DNA does not usually exist as a single strand, but instead as a pair of strands that are held tightly together.[9][12]These two long strands coil around each other, in the shape of adouble helix.The nucleotide contains both a segment of thebackboneof the molecule (which holds the chain together) and anucleobase(which interacts with the other DNA strand in the helix). A nucleobase linked to a sugar is called anucleoside,and a base linked to a sugar and to one or more phosphate groups is called anucleotide.Abiopolymercomprising multiple linked nucleotides (as in DNA) is called apolynucleotide.[13]
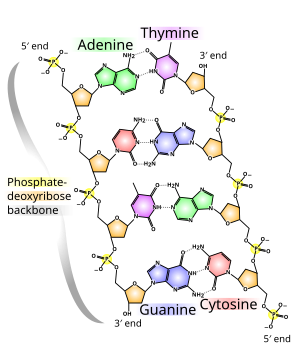
The backbone of the DNA strand is made from alternatingphosphateandsugargroups.[14]The sugar in DNA is2-deoxyribose,which is apentose(five-carbon) sugar. The sugars are joined by phosphate groups that formphosphodiester bondsbetween the third and fifth carbonatomsof adjacent sugar rings. These are known as the3′-end(three prime end), and5′-end(five prime end) carbons, the prime symbol being used to distinguish these carbon atoms from those of the base to which the deoxyribose forms aglycosidic bond.[12]
Therefore, any DNA strand normally has one end at which there is a phosphate group attached to the 5′ carbon of a ribose (the 5′ phosphoryl) and another end at which there is a free hydroxyl group attached to the 3′ carbon of a ribose (the 3′ hydroxyl). The orientation of the 3′ and 5′ carbons along the sugar-phosphate backbone confersdirectionality(sometimes called polarity) to each DNA strand. In anucleic acid double helix,the direction of the nucleotides in one strand is opposite to their direction in the other strand: the strands areantiparallel.The asymmetric ends of DNA strands are said to have a directionality of five prime end (5′ ), and three prime end (3′), with the 5′ end having a terminal phosphate group and the 3′ end a terminal hydroxyl group. One major difference between DNA andRNAis the sugar, with the 2-deoxyribose in DNA being replaced by the related pentose sugarribosein RNA.[12]
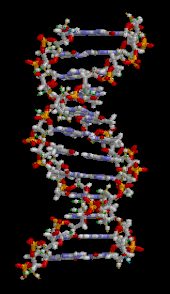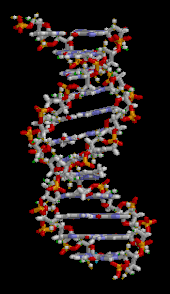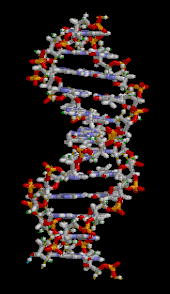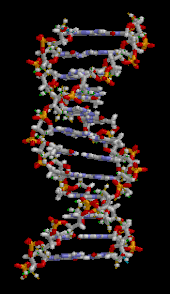
The DNA double helix is stabilized primarily by two forces:hydrogen bondsbetween nucleotides andbase-stackinginteractions amongaromaticnucleobases.[16]The four bases found in DNA areadenine(A),cytosine(C),guanine(G) andthymine(T). These four bases are attached to the sugar-phosphate to form the complete nucleotide, as shown foradenosine monophosphate.Adenine pairs with thymine and guanine pairs with cytosine, formingA-TandG-Cbase pairs.[17][18]
Nucleobase classification
The nucleobases are classified into two types: thepurines,AandG,which are fused five- and six-memberedheterocyclic compounds,and thepyrimidines,the six-membered ringsCandT.[12]A fifth pyrimidine nucleobase,uracil(U), usually takes the place of thymine in RNA and differs from thymine by lacking amethyl groupon its ring. In addition to RNA and DNA, many artificialnucleic acid analogueshave been created to study the properties of nucleic acids, or for use in biotechnology.[19]
Non-canonical bases
Modified bases occur in DNA. The first of these recognized was5-methylcytosine,which was found in thegenomeofMycobacterium tuberculosisin 1925.[20]The reason for the presence of these noncanonical bases in bacterial viruses (bacteriophages) is to avoid therestriction enzymespresent in bacteria. This enzyme system acts at least in part as a molecular immune system protecting bacteria from infection by viruses.[21]Modifications of the bases cytosine and adenine, the more common and modified DNA bases, play vital roles in theepigeneticcontrol of gene expression in plants and animals.[22]
A number of noncanonical bases are known to occur in DNA.[23]Most of these are modifications of the canonical bases plus uracil.
- ModifiedAdenine
- N6-carbamoyl-methyladenine
- N6-methyadenine
- ModifiedGuanine
- 7-Deazaguanine
- 7-Methylguanine
- ModifiedCytosine
- N4-Methylcytosine
- 5-Carboxylcytosine
- 5-Formylcytosine
- 5-Glycosylhydroxymethylcytosine
- 5-Hydroxycytosine
- 5-Methylcytosine
- ModifiedThymidine
- α-Glutamythymidine
- α-Putrescinylthymine
- Uraciland modifications
- Base J
- Uracil
- 5-Dihydroxypentauracil
- 5-Hydroxymethyldeoxyuracil
- Others
- Deoxyarchaeosine
- 2,6-Diaminopurine (2-Aminoadenine)
Grooves
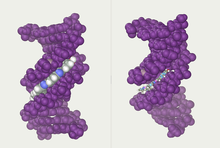
Twin helical strands form the DNA backbone. Another double helix may be found tracing the spaces, or grooves, between the strands. These voids are adjacent to the base pairs and may provide abinding site.As the strands are not symmetrically located with respect to each other, the grooves are unequally sized. The major groove is 22 ångströms (2.2 nm) wide, while the minor groove is 12 Å (1.2 nm) in width.[24]Due to the larger width of the major groove, the edges of the bases are more accessible in the major groove than in the minor groove. As a result, proteins such astranscription factorsthat can bind to specific sequences in double-stranded DNA usually make contact with the sides of the bases exposed in the major groove.[25]This situation varies in unusual conformations of DNA within the cell(see below),but the major and minor grooves are always named to reflect the differences in width that would be seen if the DNA was twisted back into the ordinaryB form.
Base pairing

|
|
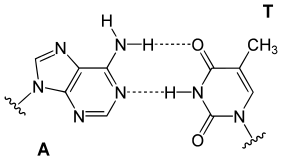
In a DNA double helix, each type of nucleobase on one strand bonds with just one type of nucleobase on the other strand. This is calledcomplementarybase pairing.Purines formhydrogen bondsto pyrimidines, with adenine bonding only to thymine in two hydrogen bonds, and cytosine bonding only to guanine in three hydrogen bonds. This arrangement of two nucleotides binding together across the double helix (from six-carbon ring to six-carbon ring) is called a Watson-Crick base pair. DNA with highGC-contentis more stable than DNA with lowGC-content. AHoogsteen base pair(hydrogen bonding the 6-carbon ring to the 5-carbon ring) is a rare variation of base-pairing.[26]As hydrogen bonds are notcovalent,they can be broken and rejoined relatively easily. The two strands of DNA in a double helix can thus be pulled apart like a zipper, either by a mechanical force or hightemperature.[27]As a result of this base pair complementarity, all the information in the double-stranded sequence of a DNA helix is duplicated on each strand, which is vital in DNA replication. This reversible and specific interaction between complementary base pairs is critical for all the functions of DNA in organisms.[7]
ssDNA vs. dsDNA
Most DNA molecules are actually two polymer strands, bound together in a helical fashion by noncovalent bonds; this double-stranded (dsDNA) structure is maintained largely by the intrastrand base stacking interactions, which are strongest forG,Cstacks. The two strands can come apart—a process known as melting—to form two single-stranded DNA (ssDNA) molecules. Melting occurs at high temperatures, low salt and highpH(low pH also melts DNA, but since DNA is unstable due to acid depurination, low pH is rarely used).
The stability of the dsDNA form depends not only on theGC-content (%G,Cbasepairs) but also on sequence (since stacking is sequence specific) and also length (longer molecules are more stable). The stability can be measured in various ways; a common way is themelting temperature(also calledTmvalue), which is the temperature at which 50% of the double-strand molecules are converted to single-strand molecules; melting temperature is dependent on ionic strength and the concentration of DNA. As a result, it is both the percentage ofGCbase pairs and the overall length of a DNA double helix that determines the strength of the association between the two strands of DNA. Long DNA helices with a highGC-content have more strongly interacting strands, while short helices with highATcontent have more weakly interacting strands.[28]In biology, parts of the DNA double helix that need to separate easily, such as theTATAATPribnow boxin somepromoters,tend to have a highATcontent, making the strands easier to pull apart.[29]
In the laboratory, the strength of this interaction can be measured by finding the melting temperatureTmnecessary to break half of the hydrogen bonds. When all the base pairs in a DNA double helix melt, the strands separate and exist in solution as two entirely independent molecules. These single-stranded DNA molecules have no single common shape, but some conformations are more stable than others.[30]
Amount

In humans, the total femalediploidnuclear genomeper cell extends for 6.37 Gigabase pairs (Gbp), is 208.23 cm long and weighs 6.51 picograms (pg).[31]Male values are 6.27 Gbp, 205.00 cm, 6.41 pg.[31]Each DNA polymer can contain hundreds of millions of nucleotides, such as inchromosome 1.Chromosome 1 is the largest humanchromosomewith approximately 220 millionbase pairs,and would be85 mmlong if straightened.[32]
Ineukaryotes,in addition tonuclear DNA,there is alsomitochondrial DNA(mtDNA) which encodes certain proteins used by the mitochondria. The mtDNA is usually relatively small in comparison to the nuclear DNA. For example, thehuman mitochondrial DNAforms closed circular molecules, each of which contains 16,569[33][34]DNA base pairs,[35]with each such molecule normally containing a full set of the mitochondrial genes. Each human mitochondrion contains, on average, approximately 5 such mtDNA molecules.[35]Each humancellcontains approximately 100 mitochondria, giving a total number of mtDNA molecules per human cell of approximately 500.[35]However, the amount of mitochondria per cell also varies by cell type, and anegg cellcan contain 100,000 mitochondria, corresponding to up to 1,500,000 copies of the mitochondrial genome (constituting up to 90% of the DNA of the cell).[36]
Sense and antisense
ADNA sequenceis called a "sense" sequence if it is the same as that of amessenger RNAcopy that is translated into protein.[37]The sequence on the opposite strand is called the "antisense" sequence. Both sense and antisense sequences can exist on different parts of the same strand of DNA (i.e. both strands can contain both sense and antisense sequences). In both prokaryotes and eukaryotes, antisense RNA sequences are produced, but the functions of these RNAs are not entirely clear.[38]One proposal is that antisense RNAs are involved in regulatinggene expressionthrough RNA-RNA base pairing.[39]
A few DNA sequences in prokaryotes and eukaryotes, and more inplasmidsandviruses,blur the distinction between sense and antisense strands by havingoverlapping genes.[40]In these cases, some DNA sequences do double duty, encoding one protein when read along one strand, and a second protein when read in the opposite direction along the other strand. Inbacteria,this overlap may be involved in the regulation of gene transcription,[41]while in viruses, overlapping genes increase the amount of information that can be encoded within the small viral genome.[42]
Supercoiling
DNA can be twisted like a rope in a process calledDNA supercoiling.With DNA in its "relaxed" state, a strand usually circles the axis of the double helix once every 10.4 base pairs, but if the DNA is twisted the strands become more tightly or more loosely wound.[43]If the DNA is twisted in the direction of the helix, this is positive supercoiling, and the bases are held more tightly together. If they are twisted in the opposite direction, this is negative supercoiling, and the bases come apart more easily. In nature, most DNA has slight negative supercoiling that is introduced byenzymescalledtopoisomerases.[44]These enzymes are also needed to relieve the twisting stresses introduced into DNA strands during processes such astranscriptionandDNA replication.[45]
Alternative DNA structures
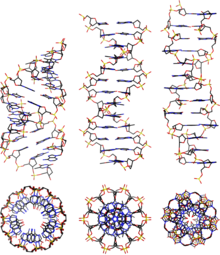
DNA exists in many possibleconformationsthat includeA-DNA,B-DNA,andZ-DNAforms, although only B-DNA and Z-DNA have been directly observed in functional organisms.[14]The conformation that DNA adopts depends on the hydration level, DNA sequence, the amount and direction of supercoiling, chemical modifications of the bases, the type and concentration of metalions,and the presence ofpolyaminesin solution.[46]
The first published reports of A-DNAX-ray diffraction patterns—and also B-DNA—used analyses based onPatterson functionsthat provided only a limited amount of structural information for oriented fibers of DNA.[47][48]An alternative analysis was proposed by Wilkinset al.in 1953 for thein vivoB-DNA X-ray diffraction-scattering patterns of highly hydrated DNA fibers in terms of squares ofBessel functions.[49]In the same journal,James WatsonandFrancis Crickpresented theirmolecular modelinganalysis of the DNA X-ray diffraction patterns to suggest that the structure was a double helix.[9]
Although theB-DNA formis most common under the conditions found in cells,[50]it is not a well-defined conformation but a family of related DNA conformations[51]that occur at the high hydration levels present in cells. Their corresponding X-ray diffraction and scattering patterns are characteristic of molecularparacrystalswith a significant degree of disorder.[52][53]
Compared to B-DNA, the A-DNA form is a widerright-handedspiral, with a shallow, wide minor groove and a narrower, deeper major groove. The A form occurs under non-physiological conditions in partly dehydrated samples of DNA, while in the cell it may be produced in hybrid pairings of DNA and RNA strands, and in enzyme-DNA complexes.[54][55]Segments of DNA where the bases have been chemically modified bymethylationmay undergo a larger change in conformation and adopt theZ form.Here, the strands turn about the helical axis in a left-handed spiral, the opposite of the more common B form.[56]These unusual structures can be recognized by specific Z-DNA binding proteins and may be involved in the regulation of transcription.[57]
Alternative DNA chemistry
For many years,exobiologistshave proposed the existence of ashadow biosphere,a postulated microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life. One of the proposals was the existence of lifeforms that usearsenic instead of phosphorus in DNA.A report in 2010 of the possibility in thebacteriumGFAJ-1was announced,[58][59]though the research was disputed,[59][60]and evidence suggests the bacterium actively prevents the incorporation of arsenic into the DNA backbone and other biomolecules.[61]
Quadruplex structures
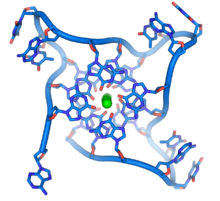
At the ends of the linear chromosomes are specialized regions of DNA calledtelomeres.The main function of these regions is to allow the cell to replicate chromosome ends using the enzymetelomerase,as the enzymes that normally replicate DNA cannot copy the extreme 3′ ends of chromosomes.[63]These specialized chromosome caps also help protect the DNA ends, and stop theDNA repairsystems in the cell from treating them as damage to be corrected.[64]Inhuman cells,telomeres are usually lengths of single-stranded DNA containing several thousand repeats of a simple TTAGGG sequence.[65]
These guanine-rich sequences may stabilize chromosome ends by forming structures of stacked sets of four-base units, rather than the usual base pairs found in other DNA molecules. Here, four guanine bases, known as aguanine tetrad,form a flat plate. These flat four-base units then stack on top of each other to form a stableG-quadruplexstructure.[66]These structures are stabilized by hydrogen bonding between the edges of the bases andchelationof a metal ion in the centre of each four-base unit.[67]Other structures can also be formed, with the central set of four bases coming from either a single strand folded around the bases, or several different parallel strands, each contributing one base to the central structure.
In addition to these stacked structures, telomeres also form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[68]At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. Thistriple-strandedstructure is called a displacement loop orD-loop.[66]
|

|
| Single branch | Multiple branches |
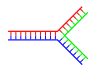
Branched DNA
In DNA,frayingoccurs when non-complementary regions exist at the end of an otherwise complementary double-strand of DNA. However, branched DNA can occur if a third strand of DNA is introduced and contains adjoining regions able to hybridize with the frayed regions of the pre-existing double-strand. Although the simplest example of branched DNA involves only three strands of DNA, complexes involving additional strands and multiple branches are also possible.[69]Branched DNA can be used innanotechnologyto construct geometric shapes, see the section onuses in technologybelow.
Artificial bases
Several artificial nucleobases have been synthesized, and successfully incorporated in the eight-base DNA analogue namedHachimoji DNA.Dubbed S, B, P, and Z, these artificial bases are capable of bonding with each other in a predictable way (S–B and P–Z), maintain the double helix structure of DNA, and be transcribed to RNA. Their existence could be seen as an indication that there is nothing special about the four natural nucleobases that evolved on Earth.[70][71]On the other hand, DNA is tightly related toRNAwhich does not only act as a transcript of DNA but also performs as molecular machines many tasks in cells. For this purpose it has to fold into a structure. It has been shown that to allow to create all possible structures at least four bases are required for the correspondingRNA,[72]while a higher number is also possible but this would be against the naturalprinciple of least effort.
Acidity
The phosphate groups of DNA give it similaracidicproperties tophosphoric acidand it can be considered as astrong acid.It will be fully ionized at a normal cellular pH, releasingprotonswhich leave behind negative charges on the phosphate groups. These negative charges protect DNA from breakdown byhydrolysisby repellingnucleophileswhich could hydrolyze it.[73]
Macroscopic appearance

Pure DNA extracted from cells forms white, stringy clumps.[74]
Chemical modifications and altered DNA packaging
|

|

|
| cytosine | 5-methylcytosine | thymine |
Base modifications and DNA packaging
The expression of genes is influenced by how the DNA is packaged in chromosomes, in a structure calledchromatin.Base modifications can be involved in packaging, with regions that have low or no gene expression usually containing high levels ofmethylationofcytosinebases. DNA packaging and its influence on gene expression can also occur by covalent modifications of thehistoneprotein core around which DNA is wrapped in the chromatin structure or else by remodeling carried out by chromatin remodeling complexes (seeChromatin remodeling). There is, further,crosstalkbetween DNA methylation and histone modification, so they can coordinately affect chromatin and gene expression.[75]
For one example, cytosine methylation produces5-methylcytosine,which is important forX-inactivationof chromosomes.[76]The average level of methylation varies between organisms—the wormCaenorhabditis eleganslacks cytosine methylation, whilevertebrateshave higher levels, with up to 1% of their DNA containing 5-methylcytosine.[77]Despite the importance of 5-methylcytosine, it candeaminateto leave a thymine base, so methylated cytosines are particularly prone tomutations.[78]Other base modifications include adenine methylation in bacteria, the presence of5-hydroxymethylcytosinein thebrain,[79]and theglycosylationof uracil to produce the "J-base" inkinetoplastids.[80][81]
Damage
DNA can be damaged by many sorts ofmutagens,which change theDNA sequence.Mutagens includeoxidizing agents,alkylating agentsand also high-energyelectromagnetic radiationsuch asultravioletlight andX-rays.The type of DNA damage produced depends on the type of mutagen. For example, UV light can damage DNA by producingthymine dimers,which are cross-links between pyrimidine bases.[83]On the other hand, oxidants such asfree radicalsorhydrogen peroxideproduce multiple forms of damage, including base modifications, particularly of guanosine, and double-strand breaks.[84]A typical human cell contains about 150,000 bases that have suffered oxidative damage.[85]Of these oxidative lesions, the most dangerous are double-strand breaks, as these are difficult to repair and can producepoint mutations,insertions,deletionsfrom the DNA sequence, andchromosomal translocations.[86]These mutations can causecancer.Because of inherent limits in the DNA repair mechanisms, if humans lived long enough, they would all eventually develop cancer.[87][88]DNA damages that arenaturally occurring,due to normal cellular processes that produce reactive oxygen species, the hydrolytic activities of cellular water, etc., also occur frequently. Although most of these damages are repaired, in any cell some DNA damage may remain despite the action of repair processes. These remaining DNA damages accumulate with age in mammalian postmitotic tissues. This accumulation appears to be an important underlying cause of aging.[89][90][91]
Many mutagens fit into the space between two adjacent base pairs, this is calledintercalation.Most intercalators arearomaticand planar molecules; examples includeethidium bromide,acridines,daunomycin,anddoxorubicin.For an intercalator to fit between base pairs, the bases must separate, distorting the DNA strands by unwinding of the double helix. This inhibits both transcription and DNA replication, causing toxicity and mutations.[92]As a result, DNA intercalators may becarcinogens,and in the case of thalidomide, ateratogen.[93]Others such asbenzo[a]pyrene diol epoxideandaflatoxinform DNA adducts that induce errors in replication.[94]Nevertheless, due to their ability to inhibit DNA transcription and replication, other similar toxins are also used inchemotherapyto inhibit rapidly growingcancercells.[95]
Biological functions
DNA usually occurs as linearchromosomesineukaryotes,andcircular chromosomesinprokaryotes.The set of chromosomes in a cell makes up itsgenome;thehuman genomehas approximately 3 billion base pairs of DNA arranged into 46 chromosomes.[96]The information carried by DNA is held in thesequenceof pieces of DNA calledgenes.Transmissionof genetic information in genes is achieved via complementary base pairing. For example, in transcription, when a cell uses the information in a gene, the DNA sequence is copied into a complementary RNA sequence through the attraction between the DNA and the correct RNA nucleotides. Usually, this RNA copy is then used to make a matchingprotein sequencein a process calledtranslation,which depends on the same interaction between RNA nucleotides. In an alternative fashion, a cell may copy its genetic information in a process calledDNA replication.The details of these functions are covered in other articles; here the focus is on the interactions between DNA and other molecules that mediate the function of the genome.
Genes and genomes
Genomic DNA is tightly and orderly packed in the process calledDNA condensation,to fit the small available volumes of the cell. In eukaryotes, DNA is located in thecell nucleus,with small amounts inmitochondriaandchloroplasts.In prokaryotes, the DNA is held within an irregularly shaped body in the cytoplasm called thenucleoid.[97]The genetic information in a genome is held within genes, and the complete set of this information in an organism is called itsgenotype.A gene is a unit ofheredityand is a region of DNA that influences a particular characteristic in an organism. Genes contain anopen reading framethat can be transcribed, andregulatory sequencessuch aspromotersandenhancers,which control transcription of the open reading frame.
In manyspecies,only a small fraction of the total sequence of thegenomeencodes protein. For example, only about 1.5% of the human genome consists of protein-codingexons,with over 50% of human DNA consisting of non-codingrepetitive sequences.[98]The reasons for the presence of so muchnoncoding DNAin eukaryotic genomes and the extraordinary differences ingenome size,orC-value,among species, represent a long-standing puzzle known as the "C-value Enigma".[99]However, some DNA sequences that do not code protein may still encode functionalnon-coding RNAmolecules, which are involved in theregulation of gene expression.[100]

Some noncoding DNA sequences play structural roles in chromosomes.Telomeresandcentromerestypically contain few genes but are important for the function and stability of chromosomes.[64][102]An abundant form of noncoding DNA in humans arepseudogenes,which are copies of genes that have been disabled by mutation.[103]These sequences are usually just molecularfossils,although they can occasionally serve as rawgenetic materialfor the creation of new genes through the process ofgene duplicationanddivergence.[104]
Transcription and translation
A gene is a sequence of DNA that contains genetic information and can influence thephenotypeof an organism. Within a gene, the sequence of bases along a DNA strand defines amessenger RNAsequence, which then defines one or more protein sequences. The relationship between the nucleotide sequences of genes and theamino-acidsequences of proteins is determined by the rules oftranslation,known collectively as thegenetic code.The genetic code consists of three-letter 'words' calledcodonsformed from a sequence of three nucleotides (e.g. ACT, CAG, TTT).
In transcription, the codons of a gene are copied into messenger RNA byRNA polymerase.This RNA copy is then decoded by aribosomethat reads the RNA sequence by base-pairing the messenger RNA totransfer RNA,which carries amino acids. Since there are 4 bases in 3-letter combinations, there are 64 possible codons (43combinations). These encode the twentystandard amino acids,giving most amino acids more than one possible codon. There are also three 'stop' or 'nonsense' codons signifying the end of the coding region; these are the TAG, TAA, and TGA codons, (UAG, UAA, and UGA on the mRNA).
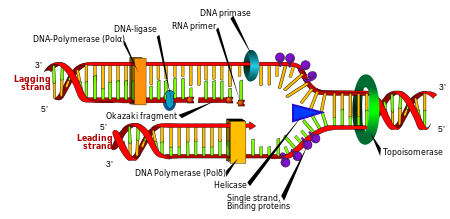
Replication
Cell divisionis essential for an organism to grow, but, when a cell divides, it must replicate the DNA in its genome so that the two daughter cells have the same genetic information as their parent. The double-stranded structure of DNA provides a simple mechanism forDNA replication.Here, the two strands are separated and then each strand'scomplementary DNAsequence is recreated by anenzymecalledDNA polymerase.This enzyme makes the complementary strand by finding the correct base through complementary base pairing and bonding it onto the original strand. As DNA polymerases can only extend a DNA strand in a 5′ to 3′ direction, different mechanisms are used to copy the antiparallel strands of the double helix.[105]In this way, the base on the old strand dictates which base appears on the new strand, and the cell ends up with a perfect copy of its DNA.
Extracellular nucleic acids
Naked extracellular DNA (eDNA), most of it released by cell death, is nearly ubiquitous in the environment. Its concentration in soil may be as high as 2 μg/L, and its concentration in natural aquatic environments may be as high at 88 μg/L.[106]Various possible functions have been proposed for eDNA: it may be involved inhorizontal gene transfer;[107]it may provide nutrients;[108]and it may act as a buffer to recruit or titrate ions or antibiotics.[109]Extracellular DNA acts as a functional extracellular matrix component in thebiofilmsof several bacterial species. It may act as a recognition factor to regulate the attachment and dispersal of specific cell types in the biofilm;[110]it may contribute to biofilm formation;[111]and it may contribute to the biofilm's physical strength and resistance to biological stress.[112]
Cell-free fetal DNAis found in the blood of the mother, and can be sequenced to determine a great deal of information about the developing fetus.[113]
Under the name ofenvironmental DNAeDNA has seen increased use in the natural sciences as a survey tool forecology,monitoring the movements and presence of species in water, air, or on land, and assessing an area's biodiversity.[114][115]
Neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA, which allowneutrophils,a type of white blood cell, to kill extracellular pathogens while minimizing damage to the host cells.
Interactions with proteins
All the functions of DNA depend on interactions with proteins. Theseprotein interactionscan be non-specific, or the protein can bind specifically to a single DNA sequence. Enzymes can also bind to DNA and of these, the polymerases that copy the DNA base sequence in transcription and DNA replication are particularly important.
DNA-binding proteins

Structural proteins that bind DNA are well-understood examples of non-specific DNA-protein interactions. Within chromosomes, DNA is held in complexes with structural proteins. These proteins organize the DNA into a compact structure calledchromatin.In eukaryotes, this structure involves DNA binding to a complex of small basic proteins calledhistones,while in prokaryotes multiple types of proteins are involved.[116][117]The histones form a disk-shaped complex called anucleosome,which contains two complete turns of double-stranded DNA wrapped around its surface. These non-specific interactions are formed through basic residues in the histones, makingionic bondsto the acidic sugar-phosphate backbone of the DNA, and are thus largely independent of the base sequence.[118]Chemical modifications of these basic amino acid residues includemethylation,phosphorylation,andacetylation.[119]These chemical changes alter the strength of the interaction between the DNA and the histones, making the DNA more or less accessible totranscription factorsand changing the rate of transcription.[120]Other non-specific DNA-binding proteins in chromatin include the high-mobility group proteins, which bind to bent or distorted DNA.[121]These proteins are important in bending arrays of nucleosomes and arranging them into the larger structures that make up chromosomes.[122]
A distinct group of DNA-binding proteins is the DNA-binding proteins that specifically bind single-stranded DNA. In humans, replicationprotein Ais the best-understood member of this family and is used in processes where the double helix is separated, including DNA replication, recombination, and DNA repair.[123]These binding proteins seem to stabilize single-stranded DNA and protect it from formingstem-loopsor being degraded bynucleases.

In contrast, other proteins have evolved to bind to particular DNA sequences. The most intensively studied of these are the varioustranscription factors,which are proteins that regulate transcription. Each transcription factor binds to one particular set of DNA sequences and activates or inhibits the transcription of genes that have these sequences close to their promoters. The transcription factors do this in two ways. Firstly, they can bind the RNA polymerase responsible for transcription, either directly or through other mediator proteins; this locates the polymerase at the promoter and allows it to begin transcription.[125]Alternatively, transcription factors can bindenzymesthat modify the histones at the promoter. This changes the accessibility of the DNA template to the polymerase.[126]
As these DNA targets can occur throughout an organism's genome, changes in the activity of one type of transcription factor can affect thousands of genes.[127]Consequently, these proteins are often the targets of thesignal transductionprocesses that control responses to environmental changes orcellular differentiationand development. The specificity of these transcription factors' interactions with DNA come from the proteins making multiple contacts to the edges of the DNA bases, allowing them to "read" the DNA sequence. Most of these base-interactions are made in the major groove, where the bases are most accessible.[25]
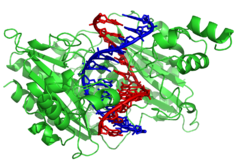
DNA-modifying enzymes
Nucleases and ligases
Nucleasesareenzymesthat cut DNA strands by catalyzing thehydrolysisof thephosphodiester bonds.Nucleases that hydrolyse nucleotides from the ends of DNA strands are calledexonucleases,whileendonucleasescut within strands. The most frequently used nucleases inmolecular biologyare therestriction endonucleases,which cut DNA at specific sequences. For instance, the EcoRV enzyme shown to the left recognizes the 6-base sequence 5′-GATATC-3′ and makes a cut at the horizontal line. In nature, these enzymes protectbacteriaagainstphageinfection by digesting the phage DNA when it enters the bacterial cell, acting as part of therestriction modification system.[129]In technology, these sequence-specific nucleases are used inmolecular cloningandDNA fingerprinting.
Enzymes calledDNA ligasescan rejoin cut or broken DNA strands.[130]Ligases are particularly important inlagging strandDNA replication, as they join the short segments of DNA produced at thereplication forkinto a complete copy of the DNA template. They are also used inDNA repairandgenetic recombination.[130]
Topoisomerases and helicases
Topoisomerasesare enzymes with both nuclease and ligase activity. These proteins change the amount ofsupercoilingin DNA. Some of these enzymes work by cutting the DNA helix and allowing one section to rotate, thereby reducing its level of supercoiling; the enzyme then seals the DNA break.[44]Other types of these enzymes are capable of cutting one DNA helix and then passing a second strand of DNA through this break, before rejoining the helix.[131]Topoisomerases are required for many processes involving DNA, such as DNA replication and transcription.[45]
Helicasesare proteins that are a type ofmolecular motor.They use the chemical energy innucleoside triphosphates,predominantlyadenosine triphosphate(ATP), to break hydrogen bonds between bases and unwind the DNA double helix into single strands.[132]These enzymes are essential for most processes where enzymes need to access the DNA bases.
Polymerases
Polymerasesareenzymesthat synthesize polynucleotide chains fromnucleoside triphosphates.The sequence of their products is created based on existing polynucleotide chains—which are calledtemplates.These enzymes function by repeatedly adding a nucleotide to the 3′hydroxylgroup at the end of the growing polynucleotide chain. As a consequence, all polymerases work in a 5′ to 3′ direction.[133]In theactive siteof these enzymes, the incoming nucleoside triphosphate base-pairs to the template: this allows polymerases to accurately synthesize the complementary strand of their template. Polymerases are classified according to the type of template that they use.
In DNA replication, DNA-dependentDNA polymerasesmake copies of DNA polynucleotide chains. To preserve biological information, it is essential that the sequence of bases in each copy are precisely complementary to the sequence of bases in the template strand. Many DNA polymerases have aproofreadingactivity. Here, the polymerase recognizes the occasional mistakes in the synthesis reaction by the lack of base pairing between the mismatched nucleotides. If a mismatch is detected, a 3′ to 5′exonucleaseactivity is activated and the incorrect base removed.[134]In most organisms, DNA polymerases function in a large complex called thereplisomethat contains multiple accessory subunits, such as theDNA clamporhelicases.[135]
RNA-dependent DNA polymerases are a specialized class of polymerases that copy the sequence of an RNA strand into DNA. They includereverse transcriptase,which is aviralenzyme involved in the infection of cells byretroviruses,andtelomerase,which is required for the replication of telomeres.[63][136]For example, HIV reverse transcriptase is an enzyme for AIDS virus replication.[136]Telomerase is an unusual polymerase because it contains its own RNA template as part of its structure. It synthesizestelomeresat the ends of chromosomes. Telomeres prevent fusion of the ends of neighboring chromosomes and protect chromosome ends from damage.[64]
Transcription is carried out by a DNA-dependentRNA polymerasethat copies the sequence of a DNA strand into RNA. To begin transcribing a gene, the RNA polymerase binds to a sequence of DNA called a promoter and separates the DNA strands. It then copies the gene sequence into amessenger RNAtranscript until it reaches a region of DNA called theterminator,where it halts and detaches from the DNA. As with human DNA-dependent DNA polymerases,RNA polymerase II,the enzyme that transcribes most of the genes in the human genome, operates as part of a largeprotein complexwith multiple regulatory and accessory subunits.[137]
Genetic recombination
|

|
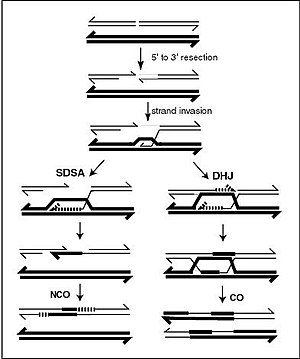
A DNA helix usually does not interact with other segments of DNA, and in human cells, the different chromosomes even occupy separate areas in the nucleus called "chromosome territories".[139]This physical separation of different chromosomes is important for the ability of DNA to function as a stable repository for information, as one of the few times chromosomes interact is inchromosomal crossoverwhich occurs duringsexual reproduction,whengenetic recombinationoccurs. Chromosomal crossover is when two DNA helices break, swap a section and then rejoin.
Recombination allows chromosomes to exchange genetic information and produces new combinations of genes, which increases the efficiency ofnatural selectionand can be important in the rapid evolution of new proteins.[140]Genetic recombination can also be involved in DNA repair, particularly in the cell's response to double-strand breaks.[141]
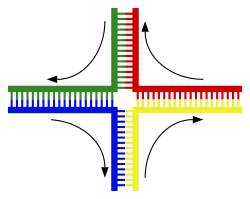
The most common form of chromosomal crossover ishomologous recombination,where the two chromosomes involved share very similar sequences.Non-homologous recombinationcan be damaging to cells, as it can producechromosomal translocationsand genetic abnormalities. The recombination reaction is catalyzed by enzymes known asrecombinases,such asRAD51.[142]The first step in recombination is a double-stranded break caused by either anendonucleaseor damage to the DNA.[143]A series of steps catalyzed in part by the recombinase then leads to joining of the two helices by at least oneHolliday junction,in which a segment of a single strand in each helix is annealed to the complementary strand in the other helix. The Holliday junction is a tetrahedral junction structure that can be moved along the pair of chromosomes, swapping one strand for another. The recombination reaction is then halted by cleavage of the junction and re-ligation of the released DNA.[144]Only strands of like polarity exchange DNA during recombination. There are two types of cleavage: east-west cleavage and north–south cleavage. The north–south cleavage nicks both strands of DNA, while the east–west cleavage has one strand of DNA intact. The formation of a Holliday junction during recombination makes it possible for genetic diversity, genes to exchange on chromosomes, and expression of wild-type viral genomes.
Evolution
DNA contains the genetic information that allows all forms of life to function, grow and reproduce. However, it is unclear how long in the 4-billion-yearhistory of lifeDNA has performed this function, as it has been proposed that the earliest forms of life may have used RNA as their genetic material.[145][146]RNA may have acted as the central part of earlycell metabolismas it can both transmit genetic information and carry outcatalysisas part ofribozymes.[147]This ancientRNA worldwhere nucleic acid would have been used for both catalysis and genetics may have influenced theevolutionof the current genetic code based on four nucleotide bases. This would occur, since the number of different bases in such an organism is a trade-off between a small number of bases increasing replication accuracy and a large number of bases increasing the catalytic efficiency of ribozymes.[148]However, there is no direct evidence of ancient genetic systems, as recovery of DNA from most fossils is impossible because DNA survives in the environment for less than one million years, and slowly degrades into short fragments in solution.[149]Claims for older DNA have been made, most notably a report of the isolation of a viable bacterium from a salt crystal 250 million years old,[150]but these claims are controversial.[151][152]
Building blocks of DNA (adenine,guanine,and relatedorganic molecules) may have been formed extraterrestrially inouter space.[153][154][155]Complex DNA andRNAorganic compoundsoflife,includinguracil,cytosine,andthymine,have also been formed in the laboratory under conditions mimicking those found inouter space,using starting chemicals, such aspyrimidine,found inmeteorites.Pyrimidine, likepolycyclic aromatic hydrocarbons(PAHs), the most carbon-rich chemical found in theuniverse,may have been formed inred giantsor in interstellarcosmic dustand gas clouds.[156]
Ancient DNAhas been recovered from ancient organisms at a timescale where genome evolution can be directly observed, including from extinct organisms up to millions of years old, such as thewoolly mammoth.[157][158]
Uses in technology
Genetic engineering
Methods have been developed to purify DNA from organisms, such asphenol-chloroform extraction,and to manipulate it in the laboratory, such asrestriction digestsand thepolymerase chain reaction.Modernbiologyandbiochemistrymake intensive use of these techniques in recombinant DNA technology.Recombinant DNAis a man-made DNA sequence that has been assembled from other DNA sequences. They can betransformedinto organisms in the form ofplasmidsor in the appropriate format, by using aviral vector.[159]Thegenetically modifiedorganisms produced can be used to produce products such as recombinantproteins,used inmedical research,[160]or be grown inagriculture.[161][162]
DNA profiling
Forensic scientistscan use DNA inblood,semen,skin,salivaorhairfound at acrime sceneto identify a matching DNA of an individual, such as a perpetrator.[163]This process is formally termedDNA profiling,also calledDNA fingerprinting.In DNA profiling, the lengths of variable sections of repetitive DNA, such asshort tandem repeatsandminisatellites,are compared between people. This method is usually an extremely reliable technique for identifying a matching DNA.[164]However, identification can be complicated if the scene is contaminated with DNA from several people.[165]DNA profiling was developed in 1984 by British geneticist SirAlec Jeffreys,[166]and first used in forensic science to convict Colin Pitchfork in the 1988Enderby murderscase.[167]
The development of forensic science and the ability to now obtain genetic matching on minute samples of blood, skin, saliva, or hair has led to re-examining many cases. Evidence can now be uncovered that was scientifically impossible at the time of the original examination. Combined with the removal of thedouble jeopardylaw in some places, this can allow cases to be reopened where prior trials have failed to produce sufficient evidence to convince a jury. People charged with serious crimes may be required to provide a sample of DNA for matching purposes. The most obvious defense to DNA matches obtained forensically is to claim that cross-contamination of evidence has occurred. This has resulted in meticulous strict handling procedures with new cases of serious crime.
DNA profiling is also used successfully to positively identify victims of mass casualty incidents,[168]bodies or body parts in serious accidents, and individual victims in mass war graves, via matching to family members.
DNA profiling is also used inDNA paternity testingto determine if someone is the biological parent or grandparent of a child with the probability of parentage is typically 99.99% when the alleged parent is biologically related to the child. NormalDNA sequencingmethods happen after birth, but there are new methods to test paternity while a mother is still pregnant.[169]
DNA enzymes or catalytic DNA
Deoxyribozymes,also called DNAzymes or catalytic DNA, were first discovered in 1994.[170]They are mostly single stranded DNA sequences isolated from a large pool of random DNA sequences through a combinatorial approach calledin vitroselection orsystematic evolution of ligands by exponential enrichment(SELEX). DNAzymes catalyze variety of chemical reactions including RNA-DNA cleavage, RNA-DNA ligation, amino acids phosphorylation-dephosphorylation, carbon-carbon bond formation, etc. DNAzymes can enhance catalytic rate of chemical reactions up to 100,000,000,000-fold over the uncatalyzed reaction.[171]The most extensively studied class of DNAzymes is RNA-cleaving types which have been used to detect different metal ions and designing therapeutic agents. Several metal-specific DNAzymes have been reported including the GR-5 DNAzyme (lead-specific),[170]the CA1-3 DNAzymes (copper-specific),[172]the 39E DNAzyme (uranyl-specific) and the NaA43 DNAzyme (sodium-specific).[173]The NaA43 DNAzyme, which is reported to be more than 10,000-fold selective for sodium over other metal ions, was used to make a real-time sodium sensor in cells.
Bioinformatics
Bioinformaticsinvolves the development of techniques to store,data mine,search and manipulate biological data, including DNAnucleic acid sequencedata. These have led to widely applied advances incomputer science,especiallystring searching algorithms,machine learning,anddatabase theory.[174]String searching or matching algorithms, which find an occurrence of a sequence of letters inside a larger sequence of letters, were developed to search for specific sequences of nucleotides.[175]The DNA sequence may bealignedwith other DNA sequences to identifyhomologous sequencesand locate the specificmutationsthat make them distinct. These techniques, especiallymultiple sequence alignment,are used in studyingphylogeneticrelationships and protein function.[176]Data sets representing entire genomes' worth of DNA sequences, such as those produced by theHuman Genome Project,are difficult to use without the annotations that identify the locations of genes and regulatory elements on each chromosome. Regions of DNA sequence that have the characteristic patterns associated with protein- or RNA-coding genes can be identified bygene findingalgorithms, which allow researchers to predict the presence of particulargene productsand their possible functions in an organism even before they have been isolated experimentally.[177]Entire genomes may also be compared, which can shed light on the evolutionary history of particular organism and permit the examination of complex evolutionary events.
DNA nanotechnology

DNA nanotechnology uses the uniquemolecular recognitionproperties of DNA and other nucleic acids to create self-assembling branched DNA complexes with useful properties.[179]DNA is thus used as a structural material rather than as a carrier of biological information. This has led to the creation of two-dimensional periodic lattices (both tile-based and using theDNA origamimethod) and three-dimensional structures in the shapes ofpolyhedra.[180]Nanomechanical devicesandalgorithmic self-assemblyhave also been demonstrated,[181]and these DNA structures have been used to template the arrangement of other molecules such asgold nanoparticlesandstreptavidinproteins.[182]DNA and other nucleic acids are the basis ofaptamers,synthetic oligonucleotide ligands for specific target molecules used in a range of biotechnology and biomedical applications.[183]
History and anthropology
Because DNA collects mutations over time, which are then inherited, it contains historical information, and, by comparing DNA sequences, geneticists can infer the evolutionary history of organisms, theirphylogeny.[184]This field of phylogenetics is a powerful tool inevolutionary biology.If DNA sequences within a species are compared,population geneticistscan learn the history of particular populations. This can be used in studies ranging fromecological geneticstoanthropology.
Information storage
DNA as astorage devicefor information has enormous potential since it has much higherstorage densitycompared to electronic devices. However, high costs, slow read and write times (memory latency), and insufficientreliabilityhas prevented its practical use.[185][186]
History

DNA was first isolated by the Swiss physicianFriedrich Miescherwho, in 1869, discovered a microscopic substance in thepusof discarded surgical bandages. As it resided in the nuclei of cells, he called it "nuclein".[187][188]In 1878,Albrecht Kosselisolated the non-protein component of "nuclein", nucleic acid, and later isolated its five primarynucleobases.[189][190]
In 1909,Phoebus Leveneidentified the base, sugar, and phosphate nucleotide unit of RNA (then named "yeast nucleic acid" ).[191][192][193]In 1929, Levene identified deoxyribose sugar in "thymus nucleic acid" (DNA).[194]Levene suggested that DNA consisted of a string of four nucleotide units linked together through the phosphate groups ( "tetranucleotide hypothesis" ). Levene thought the chain was short and the bases repeated in a fixed order. In 1927,Nikolai Koltsovproposed that inherited traits would be inherited via a "giant hereditary molecule" made up of "two mirror strands that would replicate in a semi-conservative fashion using each strand as a template".[195][196]In 1928,Frederick Griffithin hisexperimentdiscovered thattraitsof the "smooth" form ofPneumococcuscould be transferred to the "rough" form of the same bacteria by mi xing killed "smooth" bacteria with the live "rough" form.[197][198]This system provided the first clear suggestion that DNA carries genetic information.
In 1933, while studying virginsea urchineggs,Jean Brachetsuggested that DNA is found in thecell nucleusand thatRNAis present exclusively in thecytoplasm.At the time, "yeast nucleic acid" (RNA) was thought to occur only in plants, while "thymus nucleic acid" (DNA) only in animals. The latter was thought to be a tetramer, with the function of buffering cellular pH.[199][200]
In 1937,William Astburyproduced the first X-ray diffraction patterns that showed that DNA had a regular structure.[201]
In 1943,Oswald Avery,along with co-workersColin MacLeodandMaclyn McCarty,identified DNA as thetransforming principle,supporting Griffith's suggestion (Avery–MacLeod–McCarty experiment).[202]Erwin Chargaffdeveloped and published observations now known asChargaff's rules,stating that in DNA from any species of any organism, the amount ofguanineshould be equal tocytosineand the amount ofadenineshould be equal tothymine.[203][204]

Late in 1951,Francis Crickstarted working withJames Watsonat theCavendish Laboratorywithin theUniversity of Cambridge.DNA's role inhereditywas confirmed in 1952 whenAlfred HersheyandMartha Chasein theHershey–Chase experimentshowed that DNA is thegenetic materialof theenterobacteria phage T2.[205]
In May 1952,Raymond Gosling,a graduate student working under the supervision ofRosalind Franklin,took anX-ray diffractionimage, labeled as "Photo 51",[206]at high hydration levels of DNA. This photo was given to Watson and Crick byMaurice Wilkinsand was critical to their obtaining the correct structure of DNA. Franklin told Crick and Watson that the backbones had to be on the outside. Before then, Linus Pauling, and Watson and Crick, had erroneous models with the chains inside and the bases pointing outwards. Franklin's identification of thespace groupfor DNA crystals revealed to Crick that the two DNA strands wereantiparallel.[207]In February 1953,Linus PaulingandRobert Coreyproposed a model for nucleic acids containing three intertwined chains, with the phosphates near the axis, and the bases on the outside.[208]Watson and Crick completed their model, which is now accepted as the first correct model of the double helix ofDNA.On 28 February 1953 Crick interrupted patrons' lunchtime atThe Eaglepubin Cambridge, England to announce that he and Watson had "discovered the secret of life".[209]
The 25 April 1953 issue of the journalNaturepublished a series of five articles giving the Watson and Crick double-helix structure DNA and evidence supporting it.[210]The structure was reported in a letter titled "MOLECULAR STRUCTURE OF NUCLEIC ACIDS A Structure for Deoxyribose Nucleic Acid",in which they said, "It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material."[9]This letter was followed by a letter from Franklin and Gosling, which was the first publication of their own X-ray diffraction data and of their original analysis method.[48][211]Then followed a letter by Wilkins and two of his colleagues, which contained an analysis ofin vivoB-DNA X-ray patterns, and which supported the presencein vivoof the Watson and Crick structure.[49]
In April 2023, scientists, based on new evidence, concluded that Rosalind Franklin was a contributor and "equal player" in the discovery process of DNA, rather than otherwise, as may have been presented subsequently after the time of the discovery.[212][213][214]
In 1962, after Franklin's death, Watson, Crick, and Wilkins jointly received theNobel Prize in Physiology or Medicine.[215]Nobel Prizes are awarded only to living recipients. A debate continues about who should receive credit for the discovery.[216]
In an influential presentation in 1957, Crick laid out thecentral dogma of molecular biology,which foretold the relationship between DNA, RNA, and proteins, and articulated the "adaptor hypothesis".[217]Final confirmation of the replication mechanism that was implied by the double-helical structure followed in 1958 through theMeselson–Stahl experiment.[218]Further work by Crick and co-workers showed that the genetic code was based on non-overlapping triplets of bases, calledcodons,allowingHar Gobind Khorana,Robert W. Holley,andMarshall Warren Nirenbergto decipher the genetic code.[219]These findings represent the birth ofmolecular biology.[220]
In 1986, DNA analysis was first used for criminal investigative purposes when police in the UK requestedAlec Jeffreysof the University of Leicester to verify or disprove a suspect's rape-murder "confession". In this particular case, the suspect had confessed to two rape-murders, but had later retracted his confession. DNA testing at the university labs soon disproved the veracity of the suspect's original "confession", and the suspect was exonerated from the murder-rape charges.[221]
See also
- Autosome– Any chromosome other than a sex chromosome
- Crystallography– Scientific study of crystal structures
- DNA Day– Holiday celebrated on April 25
- DNA microarray– Collection of microscopic DNA spots attached to a solid surface
- DNA sequencing– Process of determining the nucleic acid sequence
- Genetic disorder– Health problem caused by one or more abnormalities in the genome
- Genetic genealogy– DNA testing to infer relationships
- Haplotype– Group of genes from one parent
- Meiosis– Cell division producing haploid gametes
- Nucleic acid notation– Universal notation using the Roman characters A, C, G, and T to call the four DNA nucleotides
- Nucleic acid sequence– Succession of nucleotides in a nucleic acid
- Ribosomal DNA– specific region of DNA that that codes for ribosomal RNA
- Southern blot– DNA analysis technique
- X-ray scattering techniques– family of non-destructive analytical techniques
- Xeno nucleic acid– Synthetic nucleic acid analogues
References
- ^"deoxyribonucleic acid".Merriam-Webster Dictionary.
- ^Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2014).Molecular Biology of the Cell(6th ed.). Garland. p. Chapter 4: DNA, Chromosomes and Genomes.ISBN978-0-8153-4432-2.Archivedfrom the original on 14 July 2014.
- ^Purcell A."DNA".Basic Biology.Archivedfrom the original on 5 January 2017.
- ^"Uracil".Genome.gov.Retrieved21 November2019.
- ^Russell P (2001).iGenetics.New York: Benjamin Cummings.ISBN0-8053-4553-1.
- ^Saenger W (1984).Principles of Nucleic Acid Structure.New York: Springer-Verlag.ISBN0-387-90762-9.
- ^abAlberts B, Johnson A, Lewis J, Raff M, Roberts K, Peter W (2002).Molecular Biology of the Cell(Fourth ed.). New York and London: Garland Science.ISBN0-8153-3218-1.OCLC145080076.Archivedfrom the original on 1 November 2016.
- ^Irobalieva RN, Fogg JM, Catanese DJ, Catanese DJ, Sutthibutpong T, Chen M, Barker AK, Ludtke SJ, Harris SA, Schmid MF, Chiu W, Zechiedrich L (October 2015)."Structural diversity of supercoiled DNA".Nature Communications.6:8440.Bibcode:2015NatCo...6.8440I.doi:10.1038/ncomms9440.ISSN2041-1723.PMC4608029.PMID26455586.
- ^abcdWatson JD, Crick FH (April 1953)."Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid"(PDF).Nature.171(4356): 737–38.Bibcode:1953Natur.171..737W.doi:10.1038/171737a0.ISSN0028-0836.PMID13054692.S2CID4253007.Archived(PDF)from the original on 4 February 2007.
- ^Mandelkern M, Elias JG, Eden D, Crothers DM (October 1981). "The dimensions of DNA in solution".Journal of Molecular Biology.152(1): 153–61.doi:10.1016/0022-2836(81)90099-1.ISSN0022-2836.PMID7338906.
- ^Arrighi, Frances E.; Mandel, Manley; Bergendahl, Janet; Hsu, T. C. (June 1970). "Buoyant densities of DNA of mammals".Biochemical Genetics.4(3): 367–376.doi:10.1007/BF00485753.PMID4991030.S2CID27950750.
- ^abcdBerg J, Tymoczko J, Stryer L (2002).Biochemistry.W.H. Freeman and Company.ISBN0-7167-4955-6.
- ^IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (December 1970)."Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents. Recommendations 1970".The Biochemical Journal.120(3): 449–54.doi:10.1042/bj1200449.ISSN0306-3283.PMC1179624.PMID5499957.Archived fromthe originalon 5 February 2007.
- ^abGhosh A, Bansal M (April 2003). "A glossary of DNA structures from A to Z".Acta Crystallographica Section D.59(Pt 4): 620–26.Bibcode:2003AcCrD..59..620G.doi:10.1107/S0907444903003251.ISSN0907-4449.PMID12657780.
- ^Edwards KJ, Brown DG, Spink N, Skelly JV, Neidle S."RCSB PDB – 1D65: Molecular structure of the B-DNA dodecamer d(CGCAAATTTGCG)2. An examination of propeller twist and minor-groove water structure at 2.2 A resolution".rcsb.org.Retrieved27 March2023.
- ^Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006)."Base-stacking and base-pairing contributions into thermal stability of the DNA double helix".Nucleic Acids Research.34(2): 564–74.doi:10.1093/nar/gkj454.ISSN0305-1048.PMC1360284.PMID16449200.
- ^Tropp BE (2012).Molecular Biology(4th ed.). Sudbury, Mass.: Jones and Barlett Learning.ISBN978-0-7637-8663-2.
- ^Carr S (1953)."Watson-Crick Structure of DNA".Memorial University of Newfoundland.Archivedfrom the original on 19 July 2016.Retrieved13 July2016.
- ^Verma S, Eckstein F (1998)."Modified oligonucleotides: synthesis and strategy for users".Annual Review of Biochemistry.67:99–134.doi:10.1146/annurev.biochem.67.1.99.ISSN0066-4154.PMID9759484.
- ^Johnson TB, Coghill RD (1925). "Pyrimidines. CIII. The discovery of 5-methylcytosine in tuberculinic acid, the nucleic acid of the tubercle bacillus".Journal of the American Chemical Society.47:2838–44.doi:10.1021/ja01688a030.ISSN0002-7863.
- ^Weigele P, Raleigh EA (October 2016)."Biosynthesis and Function of Modified Bases in Bacteria and Their Viruses".Chemical Reviews.116(20): 12655–12687.doi:10.1021/acs.chemrev.6b00114.ISSN0009-2665.PMID27319741.
- ^Kumar S, Chinnusamy V, Mohapatra T (2018)."Epigenetics of Modified DNA Bases: 5-Methylcytosine and Beyond".Frontiers in Genetics.9:640.doi:10.3389/fgene.2018.00640.ISSN1664-8021.PMC6305559.PMID30619465.
- ^Carell T, Kurz MQ, Müller M, Rossa M, Spada F (April 2018). "Non-canonical Bases in the Genome: The Regulatory Information Layer in DNA".Angewandte Chemie.57(16): 4296–4312.doi:10.1002/anie.201708228.PMID28941008.
- ^Wing R, Drew H, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE (October 1980). "Crystal structure analysis of a complete turn of B-DNA".Nature.287(5784): 755–58.Bibcode:1980Natur.287..755W.doi:10.1038/287755a0.PMID7432492.S2CID4315465.
- ^abPabo CO, Sauer RT (1984). "Protein-DNA recognition".Annual Review of Biochemistry.53:293–321.doi:10.1146/annurev.bi.53.070184.001453.PMID6236744.
- ^Nikolova EN, Zhou H, Gottardo FL, Alvey HS, Kimsey IJ, Al-Hashimi HM (2013)."A historical account of Hoogsteen base-pairs in duplex DNA".Biopolymers.99(12): 955–68.doi:10.1002/bip.22334.PMC3844552.PMID23818176.
- ^Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub HE (April 2000)."Mechanical stability of single DNA molecules".Biophysical Journal.78(4): 1997–2007.Bibcode:2000BpJ....78.1997C.doi:10.1016/S0006-3495(00)76747-6.PMC1300792.PMID10733978.
- ^Chalikian TV, Völker J, Plum GE, Breslauer KJ (July 1999)."A more unified picture for the thermodynamics of nucleic acid duplex melting: a characterization by calorimetric and volumetric techniques".Proceedings of the National Academy of Sciences of the United States of America.96(14): 7853–58.Bibcode:1999PNAS...96.7853C.doi:10.1073/pnas.96.14.7853.PMC22151.PMID10393911.
- ^deHaseth PL, Helmann JD (June 1995). "Open complex formation by Escherichia coli RNA polymerase: the mechanism of polymerase-induced strand separation of double helical DNA".Molecular Microbiology.16(5): 817–24.doi:10.1111/j.1365-2958.1995.tb02309.x.PMID7476180.S2CID24479358.
- ^Isaksson J, Acharya S, Barman J, Cheruku P, Chattopadhyaya J (December 2004)."Single-stranded adenine-rich DNA and RNA retain structural characteristics of their respective double-stranded conformations and show directional differences in stacking pattern"(PDF).Biochemistry.43(51): 15996–6010.doi:10.1021/bi048221v.PMID15609994.Archived(PDF)from the original on 10 June 2007.
- ^abPiovesan A, Pelleri MC, Antonaros F, Strippoli P, Caracausi M, Vitale L (2019)."On the length, weight and GC content of the human genome".BMC Res Notes.12(1): 106.doi:10.1186/s13104-019-4137-z.PMC6391780.PMID30813969.
- ^Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, et al. (May 2006)."The DNA sequence and biological annotation of human chromosome 1".Nature.441(7091): 315–21.Bibcode:2006Natur.441..315G.doi:10.1038/nature04727.PMID16710414.
- ^Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. (April 1981). "Sequence and organization of the human mitochondrial genome".Nature.290(5806): 457–465.Bibcode:1981Natur.290..457A.doi:10.1038/290457a0.PMID7219534.S2CID4355527.
- ^"Untitled".Archived fromthe originalon 13 August 2011.Retrieved13 June2012.
- ^abcSatoh M, Kuroiwa T (September 1991). "Organization of multiple nucleoids and DNA molecules in mitochondria of a human cell".Experimental Cell Research.196(1): 137–140.doi:10.1016/0014-4827(91)90467-9.PMID1715276.
- ^Zhang D, Keilty D, Zhang ZF, Chian RC (March 2017)."Mitochondria in oocyte aging: current understanding".Facts, Views & Vision in ObGyn.9(1): 29–38.PMC5506767.PMID28721182.
- ^Designation of the two strands of DNAArchived24 April 2008 at theWayback MachineJCBN/NC-IUB Newsletter 1989. Retrieved 7 May 2008
- ^Hüttenhofer A, Schattner P, Polacek N (May 2005). "Non-coding RNAs: hope or hype?".Trends in Genetics.21(5): 289–97.doi:10.1016/j.tig.2005.03.007.PMID15851066.
- ^Munroe SH (November 2004). "Diversity of antisense regulation in eukaryotes: multiple mechanisms, emerging patterns".Journal of Cellular Biochemistry.93(4): 664–71.doi:10.1002/jcb.20252.PMID15389973.S2CID23748148.
- ^Makalowska I, Lin CF, Makalowski W (February 2005). "Overlapping genes in vertebrate genomes".Computational Biology and Chemistry.29(1): 1–12.doi:10.1016/j pbiolchem.2004.12.006.PMID15680581.
- ^Johnson ZI, Chisholm SW (November 2004)."Properties of overlapping genes are conserved across microbial genomes".Genome Research.14(11): 2268–72.doi:10.1101/gr.2433104.PMC525685.PMID15520290.
- ^Lamb RA, Horvath CM (August 1991)."Diversity of coding strategies in influenza viruses".Trends in Genetics.7(8): 261–66.doi:10.1016/0168-9525(91)90326-L.PMC7173306.PMID1771674.
- ^Benham CJ, Mielke SP (2005)."DNA mechanics"(PDF).Annual Review of Biomedical Engineering.7:21–53.doi:10.1146/annurev.bioeng.6.062403.132016.PMID16004565.S2CID1427671.Archived fromthe original(PDF)on 1 March 2019.
- ^abChampoux JJ (2001). "DNA topoisomerases: structure, function, and mechanism".Annual Review of Biochemistry.70:369–413.doi:10.1146/annurev.biochem.70.1.369.PMID11395412.S2CID18144189.
- ^abWang JC (June 2002). "Cellular roles of DNA topoisomerases: a molecular perspective".Nature Reviews Molecular Cell Biology.3(6): 430–40.doi:10.1038/nrm831.PMID12042765.S2CID205496065.
- ^Basu HS, Feuerstein BG, Zarling DA, Shafer RH, Marton LJ (October 1988). "Recognition of Z-RNA and Z-DNA determinants by polyamines in solution: experimental and theoretical studies".Journal of Biomolecular Structure & Dynamics.6(2): 299–309.doi:10.1080/07391102.1988.10507714.PMID2482766.
- ^
- Franklin RE, Gosling RG (6 March 1953)."The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content"(PDF).Acta Crystallogr.6(8–9): 673–77.Bibcode:1953AcCry...6..673F.doi:10.1107/S0365110X53001939.Archived(PDF)from the original on 9 January 2016.
- Franklin RE, Gosling RG (1953)."The structure of sodium thymonucleate fibres. II. The cylindrically symmetrical Patterson function"(PDF).Acta Crystallogr.6(8–9): 678–85.Bibcode:1953AcCry...6..678F.doi:10.1107/S0365110X53001940.Archived(PDF)from the original on 29 June 2017.
- ^abFranklin RE, Gosling RG (April 1953)."Molecular configuration in sodium thymonucleate"(PDF).Nature.171(4356): 740–41.Bibcode:1953Natur.171..740F.doi:10.1038/171740a0.PMID13054694.S2CID4268222.Archived(PDF)from the original on 3 January 2011.
- ^abWilkins MH, Stokes AR, Wilson HR (April 1953)."Molecular structure of deoxypentose nucleic acids"(PDF).Nature.171(4356): 738–40.Bibcode:1953Natur.171..738W.doi:10.1038/171738a0.PMID13054693.S2CID4280080.Archived(PDF)from the original on 13 May 2011.
- ^Leslie AG, Arnott S, Chandrasekaran R, Ratliff RL (October 1980). "Polymorphism of DNA double helices".Journal of Molecular Biology.143(1): 49–72.doi:10.1016/0022-2836(80)90124-2.PMID7441761.
- ^Baianu IC (1980)."Structural Order and Partial Disorder in Biological systems".Bull. Math. Biol.42(4): 137–41.doi:10.1007/BF02462372.S2CID189888972.
- ^Hosemann R, Bagchi RN (1962).Direct analysis of diffraction by matter.Amsterdam – New York: North-Holland Publishers.
- ^Baianu IC (1978)."X-ray scattering by partially disordered membrane systems"(PDF).Acta Crystallogr A.34(5): 751–53.Bibcode:1978AcCrA..34..751B.doi:10.1107/S0567739478001540.Archived fromthe original(PDF)on 14 March 2020.Retrieved29 August2019.
- ^Wahl MC, Sundaralingam M (1997). "Crystal structures of A-DNA duplexes".Biopolymers.44(1): 45–63.doi:10.1002/(SICI)1097-0282(1997)44:1<45::AID-BIP4>3.0.CO;2-#.PMID9097733.
- ^Lu XJ, Shakked Z, Olson WK (July 2000). "A-form conformational motifs in ligand-bound DNA structures".Journal of Molecular Biology.300(4): 819–40.doi:10.1006/jmbi.2000.3690.PMID10891271.
- ^Rothenburg S, Koch-Nolte F, Haag F (December 2001). "DNA methylation and Z-DNA formation as mediators of quantitative differences in the expression of alleles".Immunological Reviews.184:286–98.doi:10.1034/j.1600-065x.2001.1840125.x.PMID12086319.S2CID20589136.
- ^Oh DB, Kim YG, Rich A (December 2002)."Z-DNA-binding proteins can act as potent effectors of gene expression in vivo".Proceedings of the National Academy of Sciences of the United States of America.99(26): 16666–71.Bibcode:2002PNAS...9916666O.doi:10.1073/pnas.262672699.PMC139201.PMID12486233.
- ^Palmer J (2 December 2010)."Arsenic-loving bacteria may help in hunt for alien life".BBC News.Archivedfrom the original on 3 December 2010.Retrieved2 December2010.
- ^abBortman H (2 December 2010)."Arsenic-Eating Bacteria Opens New Possibilities for Alien Life".Space.Archivedfrom the original on 4 December 2010.Retrieved2 December2010.
- ^Katsnelson A (2 December 2010)."Arsenic-eating microbe may redefine chemistry of life".Nature News.doi:10.1038/news.2010.645.Archivedfrom the original on 12 February 2012.
- ^Cressey D (3 October 2012). "'Arsenic-life' Bacterium Prefers Phosphorus after all ".Nature News.doi:10.1038/nature.2012.11520.S2CID87341731.
- ^"Structure and packing of human telomeric DNA".ndbserver.rutgers.edu.Retrieved18 May2023.
- ^abGreider CW, Blackburn EH (December 1985)."Identification of a specific telomere terminal transferase activity in Tetrahymena extracts".Cell.43(2 Pt 1): 405–13.doi:10.1016/0092-8674(85)90170-9.PMID3907856.
- ^abcNugent CI, Lundblad V (April 1998)."The telomerase reverse transcriptase: components and regulation".Genes & Development.12(8): 1073–85.doi:10.1101/gad.12.8.1073.PMID9553037.
- ^Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW (November 1997)."Normal human chromosomes have long G-rich telomeric overhangs at one end".Genes & Development.11(21): 2801–09.doi:10.1101/gad.11.21.2801.PMC316649.PMID9353250.
- ^abBurge S, Parkinson GN, Hazel P, Todd AK, Neidle S (2006)."Quadruplex DNA: sequence, topology and structure".Nucleic Acids Research.34(19): 5402–15.doi:10.1093/nar/gkl655.PMC1636468.PMID17012276.
- ^Parkinson GN, Lee MP, Neidle S (June 2002). "Crystal structure of parallel quadruplexes from human telomeric DNA".Nature.417(6891): 876–80.Bibcode:2002Natur.417..876P.doi:10.1038/nature755.PMID12050675.S2CID4422211.
- ^Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (May 1999). "Mammalian telomeres end in a large duplex loop".Cell.97(4): 503–14.CiteSeerX10.1.1.335.2649.doi:10.1016/S0092-8674(00)80760-6.PMID10338214.S2CID721901.
- ^Seeman NC (November 2005)."DNA enables nanoscale control of the structure of matter".Quarterly Reviews of Biophysics.38(4): 363–71.doi:10.1017/S0033583505004087.PMC3478329.PMID16515737.
- ^Warren M (21 February 2019)."Four new DNA letters double life's Alpha bet".Nature.566(7745): 436.Bibcode:2019Natur.566..436W.doi:10.1038/d41586-019-00650-8.PMID30809059.
- ^Hoshika S, Leal NA, Kim MJ, Kim MS, Karalkar NB, Kim HJ, et al. (22 February 2019)."Hachimoji DNA and RNA: A genetic system with eight building blocks (paywall)".Science.363(6429): 884–887.Bibcode:2019Sci...363..884H.doi:10.1126/science.aat0971.PMC6413494.PMID30792304.
- ^Burghardt B, Hartmann AK (February 2007)."RNA secondary structure design".Physical Review E.75(2): 021920.arXiv:physics/0609135.Bibcode:2007PhRvE..75b1920B.doi:10.1103/PhysRevE.75.021920.PMID17358380.S2CID17574854.
- ^Reusch W."Nucleic Acids".Michigan State University.Retrieved30 June2022.
- ^"How To Extract DNA From Anything Living".University of Utah.Retrieved30 June2022.
- ^Hu Q, Rosenfeld MG (2012)."Epigenetic regulation of human embryonic stem cells".Frontiers in Genetics.3:238.doi:10.3389/fgene.2012.00238.PMC3488762.PMID23133442.
- ^Klose RJ, Bird AP (February 2006). "Genomic DNA methylation: the mark and its mediators".Trends in Biochemical Sciences.31(2): 89–97.doi:10.1016/j.tibs.2005.12.008.PMID16403636.
- ^Bird A (January 2002)."DNA methylation patterns and epigenetic memory".Genes & Development.16(1): 6–21.doi:10.1101/gad.947102.PMID11782440.
- ^Walsh CP, Xu GL (2006). "Cytosine methylation and DNA repair".DNA Methylation: Basic Mechanisms.Current Topics in Microbiology and Immunology. Vol. 301. pp. 283–315.doi:10.1007/3-540-31390-7_11.ISBN3-540-29114-8.PMID16570853.
- ^Kriaucionis S, Heintz N (May 2009)."The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain".Science.324(5929): 929–30.Bibcode:2009Sci...324..929K.doi:10.1126/science.1169786.PMC3263819.PMID19372393.
- ^Ratel D, Ravanat JL, Berger F, Wion D (March 2006)."N6-methyladenine: the other methylated base of DNA".BioEssays.28(3): 309–15.doi:10.1002/bies.20342.PMC2754416.PMID16479578.
- ^Gommers-Ampt JH, Van Leeuwen F, de Beer AL, Vliegenthart JF, Dizdaroglu M, Kowalak JA, Crain PF, Borst P (December 1993). "beta-D-glucosyl-hydroxymethyluracil: a novel modified base present in the DNA of the parasitic protozoan T. brucei".Cell.75(6): 1129–36.doi:10.1016/0092-8674(93)90322-H.hdl:1874/5219.PMID8261512.S2CID24801094.
- ^Created fromPDB 1JDGArchived22 September 2008 at theWayback Machine
- ^Douki T, Reynaud-Angelin A, Cadet J, Sage E (August 2003). "Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation".Biochemistry.42(30): 9221–26.doi:10.1021/bi034593c.PMID12885257.
- ^Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S (March 1999). "Hydroxyl radicals and DNA base damage".Mutation Research.424(1–2): 9–21.Bibcode:1999MRFMM.424....9C.doi:10.1016/S0027-5107(99)00004-4.PMID10064846.
- ^Beckman KB, Ames BN (August 1997)."Oxidative decay of DNA".The Journal of Biological Chemistry.272(32): 19633–36.doi:10.1074/jbc.272.32.19633.PMID9289489.
- ^Valerie K, Povirk LF (September 2003)."Regulation and mechanisms of mammalian double-strand break repair".Oncogene.22(37): 5792–812.doi:10.1038/sj.onc.1206679.PMID12947387.
- ^Johnson G (28 December 2010)."Unearthing Prehistoric Tumors, and Debate".The New York Times.Archivedfrom the original on 24 June 2017.
If we lived long enough, sooner or later we all would get cancer.
- ^Alberts B, Johnson A, Lewis J, et al. (2002)."The Preventable Causes of Cancer".Molecular biology of the cell(4th ed.). New York: Garland Science.ISBN0-8153-4072-9.Archivedfrom the original on 2 January 2016.
A certain irreducible background incidence of cancer is to be expected regardless of circumstances: mutations can never be absolutely avoided, because they are an inescapable consequence of fundamental limitations on the accuracy of DNA replication, as discussed in Chapter 5. If a human could live long enough, it is inevitable that at least one of his or her cells would eventually accumulate a set of mutations sufficient for cancer to develop.
- ^Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak K (2008)."Cancer and aging as consequences of un-repaired DNA damage".In Kimura H, Suzuki A (eds.).New Research on DNA Damage.New York: Nova Science Publishers. pp. 1–47.ISBN978-1-60456-581-2.Archivedfrom the original on 25 October 2014.
- ^Hoeijmakers JH (October 2009). "DNA damage, aging, and cancer".The New England Journal of Medicine.361(15): 1475–85.doi:10.1056/NEJMra0804615.PMID19812404.
- ^Freitas AA, de Magalhães JP (2011). "A review and appraisal of the DNA damage theory of ageing".Mutation Research.728(1–2): 12–22.Bibcode:2011MRRMR.728...12F.doi:10.1016/j.mrrev.2011.05.001.PMID21600302.
- ^Ferguson LR, Denny WA (September 1991). "The genetic toxicology of acridines".Mutation Research.258(2): 123–60.doi:10.1016/0165-1110(91)90006-H.PMID1881402.
- ^Stephens TD, Bunde CJ, Fillmore BJ (June 2000). "Mechanism of action in thalidomide teratogenesis".Biochemical Pharmacology.59(12): 1489–99.doi:10.1016/S0006-2952(99)00388-3.PMID10799645.
- ^Jeffrey AM (1985). "DNA modification by chemical carcinogens".Pharmacology & Therapeutics.28(2): 237–72.doi:10.1016/0163-7258(85)90013-0.PMID3936066.
- ^Braña MF, Cacho M, Gradillas A, de Pascual-Teresa B, Ramos A (November 2001). "Intercalators as anticancer drugs".Current Pharmaceutical Design.7(17): 1745–80.doi:10.2174/1381612013397113.PMID11562309.
- ^Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (February 2001)."The sequence of the human genome".Science.291(5507): 1304–51.Bibcode:2001Sci...291.1304V.doi:10.1126/science.1058040.PMID11181995.
- ^Thanbichler M, Wang SC, Shapiro L (October 2005)."The bacterial nucleoid: a highly organized and dynamic structure".Journal of Cellular Biochemistry.96(3): 506–21.doi:10.1002/jcb.20519.PMID15988757.
- ^Wolfsberg TG, McEntyre J, Schuler GD (February 2001)."Guide to the draft human genome".Nature.409(6822): 824–26.Bibcode:2001Natur.409..824W.doi:10.1038/35057000.PMID11236998.
- ^Gregory TR (January 2005)."The C-value Enigma in plants and animals: a review of parallels and an appeal for partnership".Annals of Botany.95(1): 133–46.doi:10.1093/aob/mci009.PMC4246714.PMID15596463.
- ^Birney E,Stamatoyannopoulos JA,Dutta A, Guigó R, Gingeras TR, Margulies EH, et al. (June 2007)."Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project".Nature.447(7146): 799–816.Bibcode:2007Natur.447..799B.doi:10.1038/nature05874.PMC2212820.PMID17571346.
- ^Yin YW, Steitz TA."RCSB PDB – 1MSW: Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase".rcsb.org.Retrieved27 March2023.
- ^Pidoux AL, Allshire RC (March 2005)."The role of heterochromatin in centromere function".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.360(1455): 569–79.doi:10.1098/rstb.2004.1611.PMC1569473.PMID15905142.
- ^Harrison PM, Hegyi H, Balasubramanian S, Luscombe NM, Bertone P, Echols N, Johnson T, Gerstein M (February 2002)."Molecular fossils in the human genome: identification and analysis of the pseudogenes in chromosomes 21 and 22".Genome Research.12(2): 272–80.doi:10.1101/gr.207102.PMC155275.PMID11827946.
- ^Harrison PM, Gerstein M (May 2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution".Journal of Molecular Biology.318(5): 1155–74.doi:10.1016/S0022-2836(02)00109-2.PMID12083509.
- ^Albà M (2001)."Replicative DNA polymerases".Genome Biology.2(1): REVIEWS3002.doi:10.1186/gb-2001-2-1-reviews3002.PMC150442.PMID11178285.
- ^Tani K, Nasu M (2010). "Roles of Extracellular DNA in Bacterial Ecosystems". In Kikuchi Y, Rykova EY (eds.).Extracellular Nucleic Acids.Springer. pp.25–38.ISBN978-3-642-12616-1.
- ^Vlassov VV, Laktionov PP, Rykova EY (July 2007). "Extracellular nucleic acids".BioEssays.29(7): 654–67.doi:10.1002/bies.20604.PMID17563084.S2CID32463239.
- ^Finkel SE, Kolter R (November 2001)."DNA as a nutrient: novel role for bacterial competence gene homologs".Journal of Bacteriology.183(21): 6288–93.doi:10.1128/JB.183.21.6288-6293.2001.PMC100116.PMID11591672.
- ^Mulcahy H, Charron-Mazenod L, Lewenza S (November 2008)."Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms".PLOS Pathogens.4(11): e1000213.doi:10.1371/journal.ppat.1000213.PMC2581603.PMID19023416.
- ^Berne C, Kysela DT, Brun YV (August 2010)."A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm".Molecular Microbiology.77(4): 815–29.doi:10.1111/j.1365-2958.2010.07267.x.PMC2962764.PMID20598083.
- ^Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (February 2002). "Extracellular DNA required for bacterial biofilm formation".Science.295(5559): 1487.doi:10.1126/science.295.5559.1487.PMID11859186.
- ^Hu W, Li L, Sharma S, Wang J, McHardy I, Lux R, Yang Z, He X, Gimzewski JK, Li Y, Shi W (2012)."DNA builds and strengthens the extracellular matrix in Myxococcus xanthus biofilms by interacting with exopolysaccharides".PLOS ONE.7(12): e51905.Bibcode:2012PLoSO...751905H.doi:10.1371/journal.pone.0051905.PMC3530553.PMID23300576.
- ^Hui L, Bianchi DW (February 2013)."Recent advances in the prenatal interrogation of the human fetal genome".Trends in Genetics.29(2): 84–91.doi:10.1016/j.tig.2012.10.013.PMC4378900.PMID23158400.
- ^Foote AD, Thomsen PF, Sveegaard S, Wahlberg M, Kielgast J, Kyhn LA, et al. (2012)."Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of marine mammals".PLOS ONE.7(8): e41781.Bibcode:2012PLoSO...741781F.doi:10.1371/journal.pone.0041781.PMC3430683.PMID22952587.
- ^"Researchers Detect Land Animals Using DNA in Nearby Water Bodies".
- ^Sandman K, Pereira SL, Reeve JN (December 1998)."Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome".Cellular and Molecular Life Sciences.54(12): 1350–64.doi:10.1007/s000180050259.PMC11147202.PMID9893710.S2CID21101836.
- ^Dame RT (May 2005)."The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin".Molecular Microbiology.56(4): 858–70.doi:10.1111/j.1365-2958.2005.04598.x.PMID15853876.S2CID26965112.
- ^Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ (September 1997). "Crystal structure of the nucleosome core particle at 2.8 A resolution".Nature.389(6648): 251–60.Bibcode:1997Natur.389..251L.doi:10.1038/38444.PMID9305837.S2CID4328827.
- ^Jenuwein T, Allis CD (August 2001)."Translating the histone code"(PDF).Science.293(5532): 1074–80.doi:10.1126/science.1063127.PMID11498575.S2CID1883924.Archived(PDF)from the original on 8 August 2017.
- ^Ito T (2003). "Nucleosome Assembly and Remodeling".Protein Complexes that Modify Chromatin.Current Topics in Microbiology and Immunology. Vol. 274. pp. 1–22.doi:10.1007/978-3-642-55747-7_1.ISBN978-3-540-44208-0.PMID12596902.
- ^Thomas JO (August 2001). "HMG1 and 2: architectural DNA-binding proteins".Biochemical Society Transactions.29(Pt 4): 395–401.doi:10.1042/BST0290395.PMID11497996.
- ^Grosschedl R, Giese K, Pagel J (March 1994). "HMG domain proteins: architectural elements in the assembly of nucleoprotein structures".Trends in Genetics.10(3): 94–100.doi:10.1016/0168-9525(94)90232-1.PMID8178371.
- ^Iftode C, Daniely Y, Borowiec JA (1999). "Replication protein A (RPA): the eukaryotic SSB".Critical Reviews in Biochemistry and Molecular Biology.34(3): 141–80.doi:10.1080/10409239991209255.PMID10473346.
- ^Beamer LJ, Pabo CO."RCSB PDB – 1LMB: Refined 1.8 Å crystal structure of the lambda repressor-operator complex".rcsb.org.Retrieved27 March2023.
- ^Myers LC, Kornberg RD (2000). "Mediator of transcriptional regulation".Annual Review of Biochemistry.69:729–49.doi:10.1146/annurev.biochem.69.1.729.PMID10966474.
- ^Spiegelman BM, Heinrich R (October 2004)."Biological control through regulated transcriptional coactivators".Cell.119(2): 157–67.doi:10.1016/j.cell.2004.09.037.PMID15479634.
- ^Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B (July 2003)."A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells".Proceedings of the National Academy of Sciences of the United States of America.100(14): 8164–69.Bibcode:2003PNAS..100.8164L.doi:10.1073/pnas.1332764100.PMC166200.PMID12808131.
- ^Kostrewa D, Winkler FK."RCSB PDB – 1RVA: Mg2+ binding to the active site of EcoRV endonuclease: a crystallographic study of complexes with substrate and product DNA at 2 Å resolution".rcsb.org.Retrieved27 March2023.
- ^Bickle TA, Krüger DH (June 1993)."Biology of DNA restriction".Microbiological Reviews.57(2): 434–50.doi:10.1128/MMBR.57.2.434-450.1993.PMC372918.PMID8336674.
- ^abDoherty AJ, Suh SW (November 2000)."Structural and mechanistic conservation in DNA ligases".Nucleic Acids Research.28(21): 4051–58.doi:10.1093/nar/28.21.4051.PMC113121.PMID11058099.
- ^Schoeffler AJ, Berger JM (December 2005). "Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism".Biochemical Society Transactions.33(Pt 6): 1465–70.doi:10.1042/BST20051465.PMID16246147.
- ^Tuteja N, Tuteja R (May 2004)."Unraveling DNA helicases. Motif, structure, mechanism and function"(PDF).European Journal of Biochemistry.271(10): 1849–63.doi:10.1111/j.1432-1033.2004.04094.x.PMID15128295.
- ^Joyce CM, Steitz TA (November 1995)."Polymerase structures and function: variations on a theme?".Journal of Bacteriology.177(22): 6321–29.doi:10.1128/jb.177.22.6321-6329.1995.PMC177480.PMID7592405.
- ^Hubscher U, Maga G, Spadari S (2002)."Eukaryotic DNA polymerases"(PDF).Annual Review of Biochemistry.71:133–63.doi:10.1146/annurev.biochem.71.090501.150041.PMID12045093.S2CID26171993.Archived fromthe original(PDF)on 26 January 2021.
- ^Johnson A, O'Donnell M (2005). "Cellular DNA replicases: components and dynamics at the replication fork".Annual Review of Biochemistry.74:283–315.doi:10.1146/annurev.biochem.73.011303.073859.PMID15952889.
- ^abTarrago-Litvak L, Andréola ML, Nevinsky GA, Sarih-Cottin L, Litvak S (May 1994)."The reverse transcriptase of HIV-1: from enzymology to therapeutic intervention".FASEB Journal.8(8): 497–503.doi:10.1096/fasebj.8.8.7514143.PMID7514143.S2CID39614573.
- ^Martinez E (December 2002). "Multi-protein complexes in eukaryotic gene transcription".Plant Molecular Biology.50(6): 925–47.doi:10.1023/A:1021258713850.PMID12516863.S2CID24946189.
- ^Thorpe JH, Gale BC, Teixeira SC, Cardin CJ."RCSB PDB – 1M6G: Structural Characterisation of the Holliday Junction TCGGTACCGA".rcsb.org.Retrieved27 March2023.
- ^Cremer T, Cremer C (April 2001). "Chromosome territories, nuclear architecture and gene regulation in mammalian cells".Nature Reviews Genetics.2(4): 292–301.doi:10.1038/35066075.PMID11283701.S2CID8547149.
- ^Pál C, Papp B, Lercher MJ (May 2006). "An integrated view of protein evolution".Nature Reviews Genetics.7(5): 337–48.doi:10.1038/nrg1838.PMID16619049.S2CID23225873.
- ^O'Driscoll M, Jeggo PA (January 2006). "The role of double-strand break repair – insights from human genetics".Nature Reviews Genetics.7(1): 45–54.doi:10.1038/nrg1746.PMID16369571.S2CID7779574.
- ^Vispé S, Defais M (October 1997). "Mammalian Rad51 protein: a RecA homologue with pleiotropic functions".Biochimie.79(9–10): 587–92.doi:10.1016/S0300-9084(97)82007-X.PMID9466696.
- ^Neale MJ, Keeney S (July 2006)."Clarifying the mechanics of DNA strand exchange in meiotic recombination".Nature.442(7099): 153–58.Bibcode:2006Natur.442..153N.doi:10.1038/nature04885.PMC5607947.PMID16838012.
- ^Dickman MJ, Ingleston SM, Sedelnikova SE, Rafferty JB, Lloyd RG, Grasby JA, Hornby DP (November 2002)."The RuvABC resolvasome".European Journal of Biochemistry.269(22): 5492–501.doi:10.1046/j.1432-1033.2002.03250.x.PMID12423347.S2CID39505263.
- ^Joyce GF (July 2002). "The antiquity of RNA-based evolution".Nature.418(6894): 214–21.Bibcode:2002Natur.418..214J.doi:10.1038/418214a.PMID12110897.S2CID4331004.
- ^Orgel LE (2004). "Prebiotic chemistry and the origin of the RNA world".Critical Reviews in Biochemistry and Molecular Biology.39(2): 99–123.CiteSeerX10.1.1.537.7679.doi:10.1080/10409230490460765.PMID15217990.S2CID4939632.
- ^Davenport RJ (May 2001). "Ribozymes. Making copies in the RNA world".Science.292(5520): 1278a–1278.doi:10.1126/science.292.5520.1278a.PMID11360970.S2CID85976762.
- ^Szathmáry E (April 1992)."What is the optimum size for the genetic Alpha bet?".Proceedings of the National Academy of Sciences of the United States of America.89(7): 2614–18.Bibcode:1992PNAS...89.2614S.doi:10.1073/pnas.89.7.2614.PMC48712.PMID1372984.
- ^Lindahl T (April 1993). "Instability and decay of the primary structure of DNA".Nature.362(6422): 709–15.Bibcode:1993Natur.362..709L.doi:10.1038/362709a0.PMID8469282.S2CID4283694.
- ^Vreeland RH, Rosenzweig WD, Powers DW (October 2000). "Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal".Nature.407(6806): 897–900.Bibcode:2000Natur.407..897V.doi:10.1038/35038060.PMID11057666.S2CID9879073.
- ^Hebsgaard MB, Phillips MJ, Willerslev E (May 2005). "Geologically ancient DNA: fact or artefact?".Trends in Microbiology.13(5): 212–20.doi:10.1016/j.tim.2005.03.010.PMID15866038.
- ^Nickle DC, Learn GH, Rain MW, Mullins JI, Mittler JE (January 2002). "Curiously modern DNA for a" 250 million-year-old "bacterium".Journal of Molecular Evolution.54(1): 134–37.Bibcode:2002JMolE..54..134N.doi:10.1007/s00239-001-0025-x.PMID11734907.S2CID24740859.
- ^Callahan MP, Smith KE, Cleaves HJ, Ruzicka J, Stern JC, Glavin DP, House CH, Dworkin JP (August 2011)."Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases".Proceedings of the National Academy of Sciences of the United States of America.108(34): 13995–98.Bibcode:2011PNAS..10813995C.doi:10.1073/pnas.1106493108.PMC3161613.PMID21836052.
- ^Steigerwald J (8 August 2011)."NASA Researchers: DNA Building Blocks Can Be Made in Space".NASA.Archivedfrom the original on 23 June 2015.Retrieved10 August2011.
- ^ScienceDaily Staff (9 August 2011)."DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests".ScienceDaily.Archivedfrom the original on 5 September 2011.Retrieved9 August2011.
- ^Marlaire R (3 March 2015)."NASA Ames Reproduces the Building Blocks of Life in Laboratory".NASA.Archivedfrom the original on 5 March 2015.Retrieved5 March2015.
- ^Hunt K (17 February 2021)."World's oldest DNA sequenced from a mammoth that lived more than a million years ago".CNN News.Retrieved17 February2021.
- ^Callaway E (17 February 2021)."Million-year-old mammoth genomes shatter record for oldest ancient DNA – Permafrost-preserved teeth, up to 1.6 million years old, identify a new kind of mammoth in Siberia".Nature.590(7847): 537–538.Bibcode:2021Natur.590..537C.doi:10.1038/d41586-021-00436-x.ISSN0028-0836.PMID33597786.
- ^Goff SP, Berg P (December 1976). "Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells".Cell.9(4 PT 2): 695–705.doi:10.1016/0092-8674(76)90133-1.PMID189942.S2CID41788896.
- ^Houdebine LM (2007). "Transgenic animal models in biomedical research".Target Discovery and Validation Reviews and Protocols.Methods in Molecular Biology. Vol. 360. pp. 163–202.doi:10.1385/1-59745-165-7:163.ISBN978-1-59745-165-9.PMID17172731.
- ^Daniell H, Dhingra A (April 2002)."Multigene engineering: dawn of an exciting new era in biotechnology".Current Opinion in Biotechnology.13(2): 136–41.doi:10.1016/S0958-1669(02)00297-5.PMC3481857.PMID11950565.
- ^Job D (November 2002). "Plant biotechnology in agriculture".Biochimie.84(11): 1105–10.doi:10.1016/S0300-9084(02)00013-5.PMID12595138.
- ^Curtis C, Hereward J (29 August 2017)."From the crime scene to the courtroom: the journey of a DNA sample".The Conversation.Archivedfrom the original on 22 October 2017.Retrieved22 October2017.
- ^Collins A, Morton NE (June 1994)."Likelihood ratios for DNA identification".Proceedings of the National Academy of Sciences of the United States of America.91(13): 6007–11.Bibcode:1994PNAS...91.6007C.doi:10.1073/pnas.91.13.6007.PMC44126.PMID8016106.
- ^Weir BS, Triggs CM, Starling L, Stowell LI, Walsh KA, Buckleton J (March 1997). "Interpreting DNA mixtures".Journal of Forensic Sciences.42(2): 213–22.doi:10.1520/JFS14100J.PMID9068179.S2CID14511630.
- ^Jeffreys AJ, Wilson V, Thein SL (1985)."Individual-specific 'fingerprints' of human DNA".Nature.316(6023): 76–79.Bibcode:1985Natur.316...76J.doi:10.1038/316076a0.PMID2989708.S2CID4229883.
- ^"Colin Pitchfork".14 December 2006. Archived fromthe originalon 14 December 2006.Retrieved27 March2023.
- ^"DNA Identification in Mass Fatality Incidents".National Institute of Justice. September 2006. Archived fromthe originalon 12 November 2006.
- ^Pollack A (19 June 2012)."Before Birth, Dad's ID".The New York Times.ISSN0362-4331.Archivedfrom the original on 24 June 2017.Retrieved27 March2023.
- ^abBreaker RR, Joyce GF (December 1994). "A DNA enzyme that cleaves RNA".Chemistry & Biology.1(4): 223–29.doi:10.1016/1074-5521(94)90014-0.PMID9383394.
- ^Chandra M, Sachdeva A, Silverman SK (October 2009)."DNA-catalyzed sequence-specific hydrolysis of DNA".Nature Chemical Biology.5(10): 718–20.doi:10.1038/nchembio.201.PMC2746877.PMID19684594.
- ^Carmi N, Shultz LA, Breaker RR (December 1996)."In vitro selection of self-cleaving DNAs".Chemistry & Biology.3(12): 1039–46.doi:10.1016/S1074-5521(96)90170-2.PMID9000012.
- ^Torabi SF, Wu P, McGhee CE, Chen L, Hwang K, Zheng N, Cheng J, Lu Y (May 2015)."In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing".Proceedings of the National Academy of Sciences of the United States of America.112(19): 5903–08.Bibcode:2015PNAS..112.5903T.doi:10.1073/pnas.1420361112.PMC4434688.PMID25918425.
- ^Baldi P,Brunak S (2001).Bioinformatics: The Machine Learning Approach.MIT Press.ISBN978-0-262-02506-5.OCLC45951728.
- ^Gusfield D (15 January 1997).Algorithms on Strings, Trees, and Sequences: Computer Science and Computational Biology.Cambridge University Press.ISBN978-0-521-58519-4.
- ^Sjölander K (January 2004). "Phylogenomic inference of protein molecular function: advances and challenges".Bioinformatics.20(2): 170–79.CiteSeerX10.1.1.412.943.doi:10.1093/bioinformatics/bth021.PMID14734307.
- ^Mount DM (2004).Bioinformatics: Sequence and Genome Analysis(2nd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.ISBN0-87969-712-1.OCLC55106399.
- ^Strong M (March 2004)."Protein nanomachines".PLOS Biology.2(3): E73.doi:10.1371/journal.pbio.0020073.PMC368168.PMID15024422.S2CID13222080.
- ^Rothemund PW (March 2006)."Folding DNA to create nanoscale shapes and patterns"(PDF).Nature.440(7082): 297–302.Bibcode:2006Natur.440..297R.doi:10.1038/nature04586.PMID16541064.S2CID4316391.
- ^Andersen ES, Dong M, Nielsen MM, Jahn K, Subramani R, Mamdouh W, Golas MM, Sander B, Stark H, Oliveira CL, Pedersen JS, Birkedal V, Besenbacher F, Gothelf KV, Kjems J (May 2009). "Self-assembly of a nanoscale DNA box with a controllable lid".Nature.459(7243): 73–76.Bibcode:2009Natur.459...73A.doi:10.1038/nature07971.hdl:11858/00-001M-0000-0010-9362-B.PMID19424153.S2CID4430815.
- ^Ishitsuka Y, Ha T (May 2009). "DNA nanotechnology: a nanomachine goes live".Nature Nanotechnology.4(5): 281–82.Bibcode:2009NatNa...4..281I.doi:10.1038/nnano.2009.101.PMID19421208.
- ^Aldaye FA, Palmer AL, Sleiman HF (September 2008). "Assembling materials with DNA as the guide".Science.321(5897): 1795–99.Bibcode:2008Sci...321.1795A.doi:10.1126/science.1154533.PMID18818351.S2CID2755388.
- ^Dunn MR, Jimenez RM, Chaput JC (2017)."Analysis of aptamer discovery and technology".Nature Reviews Chemistry.1(10).doi:10.1038/s41570-017-0076.Retrieved30 June2022.
- ^Wray GA (2002)."Dating branches on the tree of life using DNA".Genome Biology.3(1): REVIEWS0001.doi:10.1186/gb-2001-3-1-reviews0001.PMC150454.PMID11806830.
- ^Panda D, Molla KA, Baig MJ, Swain A, Behera D, Dash M (May 2018)."DNA as a digital information storage device: hope or hype?".3 Biotech.8(5): 239.doi:10.1007/s13205-018-1246-7.PMC5935598.PMID29744271.
- ^Akram F, Haq IU, Ali H, Laghari AT (October 2018). "Trends to store digital data in DNA: an overview".Molecular Biology Reports.45(5): 1479–1490.doi:10.1007/s11033-018-4280-y.PMID30073589.S2CID51905843.
- ^Miescher F (1871)."Ueber die chemische Zusammensetzung der Eiterzellen"[On the chemical composition of pus cells].Medicinisch-chemische Untersuchungen(in German).4:441–60.
[p. 456]Ich habe mich daher später mit meinen Versuchen an die ganzen Kerne gehalten, die Trennung der Körper, die ich einstweilen ohne weiteres Präjudiz als lösliches und unlösliches Nuclein bezeichnen will, einem günstigeren Material überlassend.(Therefore, in my experiments I subsequently limited myself to the whole nucleus, leaving to a more favorable material the separation of the substances, that for the present, without further prejudice, I will designate as soluble and insoluble nuclear material ( "Nuclein" ))
- ^Dahm R (January 2008). "Discovering DNA: Friedrich Miescher and the early years of nucleic acid research".Human Genetics.122(6): 565–81.doi:10.1007/s00439-007-0433-0.PMID17901982.S2CID915930.
- ^See:
- Kossel A (1879)."Ueber Nucleïn der Hefe"[On nuclein in yeast].Zeitschrift für physiologische Chemie(in German).3:284–91.
- Kossel A (1880)."Ueber Nucleïn der Hefe II"[On nuclein in yeast, Part 2].Zeitschrift für physiologische Chemie(in German).4:290–95.
- Kossel A (1881)."Ueber die Verbreitung des Hypoxanthins im Thier- und Pflanzenreich"[On the distribution of hypoxanthins in the animal and plant kingdoms].Zeitschrift für physiologische Chemie(in German).5:267–71.
- Kossel A (1881). Trübne KJ (ed.).Untersuchungen über die Nucleine und ihre Spaltungsprodukte[Investigations into nuclein and its cleavage products] (in German). Strassburg, Germany. p. 19.
{{cite book}}:CS1 maint: location missing publisher (link) - Kossel A (1882)."Ueber Xanthin und Hypoxanthin"[On xanthin and hypoxanthin].Zeitschrift für physiologische Chemie.6:422–31.
- Albrect Kossel (1883)"Zur Chemie des Zellkerns"Archived17 November 2017 at theWayback Machine(On the chemistry of the cell nucleus),Zeitschrift für physiologische Chemie,7:7–22.
- Kossel A (1886)."Weitere Beiträge zur Chemie des Zellkerns"[Further contributions to the chemistry of the cell nucleus].Zeitschrift für Physiologische Chemie(in German).10:248–64.
On p. 264, Kossel remarked presciently: Der Erforschung der quantitativen Verhältnisse der vier stickstoffreichen Basen, der Abhängigkeit ihrer Menge von den physiologischen Zuständen der Zelle, verspricht wichtige Aufschlüsse über die elementaren physiologisch-chemischen Vorgänge. (The study of the quantitative relations of the four nitrogenous bases—[and] of the dependence of their quantity on the physiological states of the cell—promises important insights into the fundamental physiological-chemical processes.)
- ^Jones ME (September 1953)."Albrecht Kossel, a biographical sketch".The Yale Journal of Biology and Medicine.26(1): 80–97.PMC2599350.PMID13103145.
- ^Levene PA, Jacobs WA (1909)."Über Inosinsäure".Berichte der Deutschen Chemischen Gesellschaft(in German).42:1198–203.doi:10.1002/cber.190904201196.
- ^Levene PA, Jacobs WA (1909)."Über die Hefe-Nucleinsäure".Berichte der Deutschen Chemischen Gesellschaft(in German).42(2): 2474–78.doi:10.1002/cber.190904202148.
- ^Levene P (1919)."The structure of yeast nucleic acid".J Biol Chem.40(2): 415–24.doi:10.1016/S0021-9258(18)87254-4.
- ^Cohen JS, Portugal FH (1974)."The search for the chemical structure of DNA"(PDF).Connecticut Medicine.38(10): 551–52, 554–57.PMID4609088.
- ^Koltsov proposed that a cell's genetic information was encoded in a long chain of amino acids. See:
- Koltsov HK (12 December 1927).Физико-химические основы морфологии[The physical-chemical basis of morphology] (Speech). 3rd All-Union Meeting of Zoologist, Anatomists, and Histologists (in Russian). Leningrad, U.S.S.R.
- Reprinted in:Koltsov HK (1928). "Физико-химические основы морфологии" [The physical-chemical basis of morphology].Успехи экспериментальной биологии (Advances in Experimental Biology) series B(in Russian).7(1):?.
- Reprinted in German as:Koltzoff NK (1928). "Physikalisch-chemische Grundlagen der Morphologie" [The physical-chemical basis of morphology].Biologisches Zentralblatt(in German).48(6): 345–69.
- In 1934, Koltsov contended that the proteins that contain a cell's genetic information replicate. See:Koltzoff N (October 1934). "The structure of the chromosomes in the salivary glands of Drosophila".Science.80(2075): 312–13.Bibcode:1934Sci....80..312K.doi:10.1126/science.80.2075.312.PMID17769043.
From page 313: "I think that the size of the chromosomes in the salivary glands [of Drosophila] is determined through the multiplication ofgenonemes.By this term I designate the axial thread of the chromosome, in which the geneticists locate the linear combination of genes;… In the normal chromosome there is usually only one genoneme; before cell-division this genoneme has become divided into two strands. "
- ^Soyfer VN (September 2001). "The consequences of political dictatorship for Russian science".Nature Reviews Genetics.2(9): 723–29.doi:10.1038/35088598.PMID11533721.S2CID46277758.
- ^Griffith F (January 1928)."The Significance of Pneumococcal Types".The Journal of Hygiene.27(2): 113–59.doi:10.1017/S0022172400031879.PMC2167760.PMID20474956.
- ^Lorenz MG, Wackernagel W (September 1994)."Bacterial gene transfer by natural genetic transformation in the environment".Microbiological Reviews.58(3): 563–602.doi:10.1128/MMBR.58.3.563-602.1994.PMC372978.PMID7968924.
- ^Brachet J (1933). "Recherches sur la synthese de l'acide thymonucleique pendant le developpement de l'oeuf d'Oursin".Archives de Biologie(in Italian).44:519–76.
- ^Burian R (1994)."Jean Brachet's Cytochemical Embryology: Connections with the Renovation of Biology in France?"(PDF).In Debru C, Gayon J, Picard JF (eds.).Les sciences biologiques et médicales en France 1920–1950.Cahiers pour I'histoire de la recherche. Vol. 2. Paris: CNRS Editions. pp. 207–20.
- ^See:
- Astbury WT, Bell FO (1938)."Some recent developments in the X-ray study of proteins and related structures"(PDF).Cold Spring Harbor Symposia on Quantitative Biology.6:109–21.doi:10.1101/sqb.1938.006.01.013.Archived fromthe original(PDF)on 14 July 2014.
- Astbury WT (1947)."X-ray studies of nucleic acids".Symposia of the Society for Experimental Biology(1): 66–76.PMID20257017.Archived fromthe originalon 5 July 2014.
- ^Avery OT, Macleod CM, McCarty M (February 1944)."Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III".The Journal of Experimental Medicine.79(2): 137–158.doi:10.1084/jem.79.2.137.PMC2135445.PMID19871359.
- ^Chargaff E (June 1950). "Chemical specificity of nucleic acids and mechanism of their enzymatic degradation".Experientia.6(6): 201–209.doi:10.1007/BF02173653.PMID15421335.S2CID2522535.
- ^Kresge N, Simoni RD, Hill RL (June 2005)."Chargaff's Rules: the Work of Erwin Chargaff".Journal of Biological Chemistry.280(24): 172–174.doi:10.1016/S0021-9258(20)61522-8.
- ^Hershey AD, Chase M (May 1952)."Independent functions of viral protein and nucleic acid in growth of bacteriophage".The Journal of General Physiology.36(1): 39–56.doi:10.1085/jgp.36.1.39.PMC2147348.PMID12981234.
- ^"Pictures and Illustrations: Crystallographic photo of Sodium Thymonucleate, Type B." Photo 51. "May 1952".scarc.library.oregonstate.edu.Retrieved18 May2023.
- ^Schwartz J (2008).In pursuit of the gene: from Darwin to DNA.Cambridge, Mass.: Harvard University Press.ISBN978-0-674-02670-4.
- ^Pauling L, Corey RB (February 1953)."A Proposed Structure For The Nucleic Acids".Proceedings of the National Academy of Sciences of the United States of America.39(2): 84–97.Bibcode:1953PNAS...39...84P.doi:10.1073/pnas.39.2.84.PMC1063734.PMID16578429.
- ^Regis E (2009).What Is Life?: investigating the nature of life in the age of synthetic biology.Oxford:Oxford University Press.p. 52.ISBN978-0-19-538341-6.
- ^"Double Helix of DNA: 50 Years".Nature Archives.Archived fromthe originalon 5 April 2015.
- ^"Original X-ray diffraction image".Oregon State Library.Archivedfrom the original on 30 January 2009.Retrieved6 February2011.
- ^Burakoff M (25 April 2023)."Rosalind Franklin's role in DNA discovery gets a new twist".AP News.Retrieved25 April2023.
- ^Anthes E (25 April 2023)."Untangling Rosalind Franklin's Role in DNA Discovery, 70 Years On – Historians have long debated the role that Dr. Franklin played in identifying the double helix. A new opinion essay argues that she was an" equal contributor. "".The New York Times.Archivedfrom the original on 25 April 2023.Retrieved26 April2023.
- ^Cobb M, Comfort N (25 April 2023)."What Rosalind Franklin truly contributed to the discovery of DNA's structure – Franklin was no victim in how the DNA double helix was solved. An overlooked letter and an unpublished news article, both written in 1953, reveal that she was an equal player".Nature.616(7958): 657–660.doi:10.1038/d41586-023-01313-5.PMID37100935.S2CID258314143.
- ^"The Nobel Prize in Physiology or Medicine 1962".Nobelprize.org.
- ^Maddox B (January 2003)."The double helix and the 'wronged heroine'"(PDF).Nature.421(6921): 407–08.Bibcode:2003Natur.421..407M.doi:10.1038/nature01399.PMID12540909.S2CID4428347.Archived(PDF)from the original on 17 October 2016.
- ^Crick FH (1955).A Note for the RNA Tie Club(PDF)(Speech). Cambridge, England. Archived fromthe original(PDF)on 1 October 2008.
- ^Meselson M, Stahl FW (July 1958)."The Replication of DNA in Escherichia Coli".Proceedings of the National Academy of Sciences of the United States of America.44(7): 671–82.Bibcode:1958PNAS...44..671M.doi:10.1073/pnas.44.7.671.PMC528642.PMID16590258.
- ^"The Nobel Prize in Physiology or Medicine 1968".Nobelprize.org.
- ^Pray L (2008). "Discovery of DNA structure and function: Watson and Crick".Nature Education.1(1): 100.
- ^Panneerchelvam S, Norazmi MN (2003)."Forensic DNA Profiling and Database".The Malaysian Journal of Medical Sciences.10(2): 20–26.PMC3561883.PMID23386793.
Further reading
- Berry A,Watson J(2003).DNA: the secret of life.New York: Alfred A. Knopf.ISBN0-375-41546-7.
- Calladine CR, Drew HR, Luisi BF, Travers AA (2003).Understanding DNA: the molecule & how it works.Amsterdam: Elsevier Academic Press.ISBN0-12-155089-3.
- Carina D, Clayton J (2003).50 years of DNA.Basingstoke: Palgrave Macmillan.ISBN1-4039-1479-6.
- Judson HF(1979).The Eighth Day of Creation: Makers of the Revolution in Biology(2nd ed.). Cold Spring Harbor Laboratory Press.ISBN0-671-22540-5.
- Olby RC(1994).The path to the double helix: the discovery of DNA.New York: Dover Publications.ISBN0-486-68117-3.First published in October 1974 by MacMillan, with foreword by Francis Crick; the definitive DNA textbook, revised in 1994 with a nine-page postscript.
- Olby R(January 2003)."Quiet debut for the double helix".Nature.421(6921): 402–05.Bibcode:2003Natur.421..402O.doi:10.1038/nature01397.PMID12540907.
- Olby RC (2009).Francis Crick: A Biography.Plainview, N.Y: Cold Spring Harbor Laboratory Press.ISBN978-0-87969-798-3.
- Micklas D (2003).DNA Science: A First Course.Cold Spring Harbor Press.ISBN978-0-87969-636-8.
- Ridley M(2006).Francis Crick: discoverer of the genetic code.Ashland, OH: Eminent Lives, Atlas Books.ISBN0-06-082333-X.
- Rosenfeld I (2010).DNA: A Graphic Guide to the Molecule that Shook the World.Columbia University Press.ISBN978-0-231-14271-7.
- Schultz M, Cannon Z (2009).The Stuff of Life: A Graphic Guide to Genetics and DNA.Hill and Wang.ISBN978-0-8090-8947-5.
- Stent GS,Watson J (1980).The Double Helix: A Personal Account of the Discovery of the Structure of DNA.New York: Norton.ISBN0-393-95075-1.
- Watson J (2004).DNA: The Secret of Life.Random House.ISBN978-0-09-945184-6.
- Wilkins M(2003).The third man of the double helix the autobiography of Maurice Wilkins.Cambridge, England: University Press.ISBN0-19-860665-6.
External links
- DNAatCurlie
- DNA binding site prediction on protein
- DNA the Double Helix GameFrom the official Nobel Prize web site
- DNA under electron microscope
- Dolan DNA Learning Center
- Double Helix: 50 years of DNA,Nature
- ProteopediaDNA
- ProteopediaForms_of_DNA
- ENCODE threads explorerENCODE home page atNature
- Double Helix 1953–2003National Centre for Biotechnology Education
- Genetic Education Modules for Teachers–DNA from the BeginningStudy Guide
- PDBMolecule of the MonthDNA
- "Clue to chemistry of heredity found".The New York Times,June 1953. First American newspaper coverage of the discovery of the DNA structure
- DNA from the BeginningAnother DNA Learning Center site on DNA, genes, and heredity from Mendel to the human genome project.
- The Register of Francis Crick Personal Papers 1938 – 2007at Mandeville Special Collections Library,University of California, San Diego
- Seven-page, handwritten letter that Crick sent to his 12-year-old son Michael in 1953 describing the structure of DNA.SeeCrick's medal goes under the hammer,Nature, 5 April 2013.

