Radioactive decay
| Nuclear physics |
|---|
 |
Radioactive decay(also known asnuclear decay,radioactivity,radioactive disintegration,ornuclear disintegration) is the process by which an unstableatomic nucleusloses energy byradiation.A material containing unstable nuclei is consideredradioactive.Three of the most common types of decay areAlpha,beta,andgamma decay.Theweak forceis themechanismthat is responsible for beta decay, while the other two are governed by theelectromagnetismandnuclear force.[1]
Radioactive decay is arandomprocess at the level of single atoms. According toquantum theory,it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed.[2][3][4]However, for a significant number of identical atoms, the overall decay rate can be expressed as adecay constantor ashalf-life.The half-lives of radioactive atoms have a huge range; from nearly instantaneous to far longer than theage of the universe.
The decaying nucleus is called the parentradionuclide(or parent radioisotope[note 1]), and the process produces at least onedaughter nuclide.Except for gamma decay orinternal conversionfrom a nuclearexcited state,the decay is anuclear transmutationresulting in a daughter containing a different number ofprotonsorneutrons(or both). When the number of protons changes, an atom of a differentchemical elementis created.
There are 28 naturally occurring chemical elements on Earth that are radioactive, consisting of 35radionuclides(seven elements have two different radionuclides) that date before the time of formation of theSolar System.These 35 are known asprimordial radionuclides.Well-known examples areuraniumandthorium,but also included are naturally occurring long-lived radioisotopes, such aspotassium-40.
History of discovery
[edit]
Radioactivity was discovered in 1896 by scientistsHenri BecquerelandMarie Curie,while working withphosphorescentmaterials.[5][6][7][8][9]These materials glow in the dark after exposure to light, and Becquerel suspected that the glow produced incathode ray tubesbyX-raysmight be associated with phosphorescence. He wrapped a photographic plate in black paper and placed various phosphorescentsaltson it. All results were negative until he useduraniumsalts. The uranium salts caused a blackening of the plate in spite of the plate being wrapped in black paper. These radiations were given the name "Becquerel Rays".
It soon became clear that the blackening of the plate had nothing to do with phosphorescence, as the blackening was also produced by non-phosphorescentsaltsof uranium and by metallic uranium. It became clear from these experiments that there was a form of invisible radiation that could pass through paper and was causing the plate to react as if exposed to light.
At first, it seemed as though the new radiation was similar to the then recently discovered X-rays. Further research by Becquerel,Ernest Rutherford,Paul Villard,Pierre Curie,Marie Curie, and others showed that this form of radioactivity was significantly more complicated. Rutherford was the first to realize that all such elements decay in accordance with the same mathematical exponential formula. Rutherford and his studentFrederick Soddywere the first to realize that many decay processes resulted in thetransmutationof one element to another. Subsequently, theradioactive displacement law of Fajans and Soddywas formulated to describe the products of Alpha andbeta decay.[10][11]
The early researchers also discovered that many otherchemical elements,besides uranium, have radioactive isotopes. A systematic search for the total radioactivity in uranium ores also guided Pierre and Marie Curie to isolate two new elements:poloniumandradium.Except for the radioactivity of radium, the chemical similarity of radium tobariummade these two elements difficult to distinguish.
Marie and Pierre Curie's study of radioactivity is an important factor in science and medicine. After their research on Becquerel's rays led them to the discovery of both radium and polonium, they coined the term "radioactivity"[12]to define the emission ofionizing radiationby some heavy elements.[13](Later the term was generalized to all elements.) Their research on the penetrating rays in uranium and the discovery of radium launched an era of using radium for the treatment of cancer. Their exploration of radium could be seen as the first peaceful use of nuclear energy and the start of modernnuclear medicine.[12]
Early health dangers
[edit]
The dangers ofionizing radiationdue to radioactivity and X-rays were not immediately recognized.
X-rays
[edit]The discovery of X‑rays byWilhelm Röntgenin 1895 led to widespread experimentation by scientists, physicians, and inventors. Many people began recounting stories of burns, hair loss and worse in technical journals as early as 1896. In February of that year, Professor Daniel and Dr. Dudley ofVanderbilt Universityperformed an experiment involving X-raying Dudley's head that resulted in his hair loss. A report by Dr. H.D. Hawks, of his suffering severe hand and chest burns in an X-ray demonstration, was the first of many other reports inElectrical Review.[14]
Other experimenters, includingElihu ThomsonandNikola Tesla,also reported burns. Thomson deliberately exposed a finger to an X-ray tube over a period of time and suffered pain, swelling, and blistering.[15]Other effects, including ultraviolet rays and ozone, were sometimes blamed for the damage,[16]and many physicians still claimed that there were no effects from X-ray exposure at all.[15]
Despite this, there were some early systematic hazard investigations, and as early as 1902William Herbert Rollinswrote almost despairingly that his warnings about the dangers involved in the careless use of X-rays were not being heeded, either by industry or by his colleagues. By this time, Rollins had proved that X-rays could kill experimental animals, could cause a pregnant guinea pig to abort, and that they could kill a foetus. He also stressed that "animals vary in susceptibility to the external action of X-light" and warned that these differences be considered when patients were treated by means of X-rays.[citation needed]
Radioactive substances
[edit]
However, the biological effects of radiation due to radioactive substances were less easy to gauge. This gave the opportunity for many physicians and corporations to market radioactive substances aspatent medicines.Examples were radiumenematreatments, and radium-containing waters to be drunk as tonics. Marie Curie protested against this sort of treatment, warning that "radium is dangerous in untrained hands".[17]Curie later died fromaplastic anaemia,likely caused by exposure to ionizing radiation. By the 1930s, after a number of cases of bone necrosis and death of radium treatment enthusiasts, radium-containing medicinal products had been largely removed from the market (radioactive quackery).
Radiation protection
[edit]Only a year afterRöntgen's discovery of X-rays, the American engineerWolfram Fuchs(1896) gave what is probably the first protection advice, but it was not until 1925 that the firstInternational Congress of Radiology(ICR) was held and considered establishing international protection standards. The effects of radiation on genes, including the effect of cancer risk, were recognized much later. In 1927,Hermann Joseph Mullerpublished research showing genetic effects and, in 1946, was awarded theNobel Prize in Physiology or Medicinefor his findings.
The second ICR was held in Stockholm in 1928 and proposed the adoption of theröntgenunit, and theInternational X-ray and Radium Protection Committee(IXRPC) was formed.Rolf Sievertwas named chairman, but a driving force wasGeorge Kayeof the BritishNational Physical Laboratory.The committee met in 1931, 1934, and 1937.
AfterWorld War II,the increased range and quantity of radioactive substances being handled as a result of military and civil nuclear programs led to large groups of occupational workers and the public being potentially exposed to harmful levels of ionising radiation. This was considered at the first post-war ICR convened in London in 1950, when the presentInternational Commission on Radiological Protection(ICRP) was born.[18] Since then the ICRP has developed the present international system of radiation protection, covering all aspects of radiation hazards.
In 2020, Hauptmann and another 15 international researchers from eight nations, among them: Institutes of Biostatistics, Registry Research, Centers of Cancer Epidemiology, Radiation Epidemiology, and also theU.S. National Cancer Institute(NCI),International Agency for Research on Cancer(IARC) and theRadiation Effects Research Foundation of Hiroshimastudied definitively throughmeta-analysisthe damage resulting from the "low doses" that have afflicted survivors of theatomic bombings of Hiroshima and Nagasakiand also in numerousaccidents at nuclear plantsthat have occurred. These scientists reported, inJNCI Monographs: Epidemiological Studies of Low Dose Ionizing Radiation and Cancer Risk,that the new epidemiological studies directly support excess cancer risks from low-dose ionizing radiation.[19]In 2021, Italian researcher Sebastiano Venturi reported the first correlations between radio-caesium andpancreatic cancerwith the role ofcaesiumin biology, in pancreatitis and in diabetes of pancreatic origin.[20]
Units
[edit]
TheInternational System of Units(SI) unit of radioactive activity is thebecquerel(Bq), named in honor of the scientistHenri Becquerel.One Bq is defined as one transformation (or decay or disintegration) per second.
An older unit of radioactivity is thecurie,Ci, which was originally defined as "the quantity or mass ofradium emanationinequilibriumwith one gram ofradium(element) ".[21]Today, the curie is defined as3.7×1010disintegrations per second, so that 1curie(Ci) =3.7×1010Bq. For radiological protection purposes, although the United States Nuclear Regulatory Commission permits the use of the unit curie alongside SI units,[22]theEuropean UnionEuropean units of measurement directivesrequired that its use for "public health... purposes" be phased out by 31 December 1985.[23]
The effects of ionizing radiation are often measured in units ofgrayfor mechanical orsievertfor damage to tissue.
Types
[edit]This sectionneeds additional citations forverification.(May 2023) |
Radioactive decay results in a reduction of summed restmass,once the released energy (thedisintegration energy) has escaped in some way. Althoughdecay energyis sometimes defined as associated with the difference between the mass of the parent nuclide products and the mass of the decay products, this is true only of rest mass measurements, where some energy has been removed from the product system. This is true because the decay energy must always carry mass with it, wherever it appears (seemass in special relativity) according to the formulaE=mc2.The decay energy is initially released as the energy of emitted photons plus the kinetic energy of massive emitted particles (that is, particles that have rest mass). If these particles come tothermal equilibriumwith their surroundings and photons are absorbed, then the decay energy is transformed to thermal energy, which retains its mass.
Decay energy, therefore, remains associated with a certain measure of the mass of the decay system, calledinvariant mass,which does not change during the decay, even though the energy of decay is distributed among decay particles. The energy of photons, the kinetic energy of emitted particles, and, later, the thermal energy of the surrounding matter, all contribute to theinvariant massof the system. Thus, while the sum of the rest masses of the particles is not conserved in radioactive decay, thesystemmass and system invariant mass (and also the system total energy) is conserved throughout any decay process. This is a restatement of the equivalent laws ofconservation of energyandconservation of mass.
Alpha, beta and gamma decay
[edit]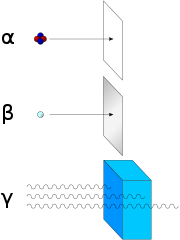
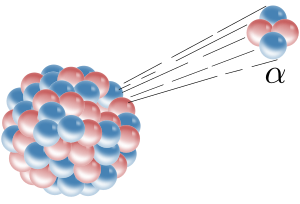
Early researchers found that anelectricormagnetic fieldcould split radioactive emissions into three types of beams. The rays were given the namesAlpha,beta,and gamma, in increasing order of their ability to penetrate matter. Alpha decay is observed only in heavier elements of atomic number 52 (tellurium) and greater, with the exception ofberyllium-8(which decays to two Alpha particles). The other two types of decay are observed in all the elements. Lead,atomic number82, is the heaviest element to have any isotopes stable (to the limit of measurement) to radioactive decay. Radioactive decay is seen in all isotopes of all elements of atomic number 83 (bismuth) or greater.Bismuth-209,however, is only very slightly radioactive, with a half-life greater than the age of the universe; radioisotopes with extremely long half-lives are considered effectively stable for practical purposes.
In analyzing the nature of the decay products, it was obvious from the direction of theelectromagnetic forcesapplied to the radiations by external magnetic and electric fields that Alpha particles carried a positive charge, beta particles carried a negative charge, and gamma rays were neutral. From the magnitude of deflection, it was clear thatAlpha particleswere much more massive thanbeta particles.Passing Alpha particles through a very thin glass window and trapping them in adischarge tubeallowed researchers to study theemission spectrumof the captured particles, and ultimately proved that Alpha particles areheliumnuclei. Other experiments showed beta radiation, resulting from decay andcathode rays,were high-speedelectrons.Likewise, gamma radiation and X-rays were found to be high-energyelectromagnetic radiation.
The relationship between the types of decays also began to be examined: For example, gamma decay was almost always found to be associated with other types of decay, and occurred at about the same time, or afterwards. Gamma decay as a separate phenomenon, with its own half-life (now termedisomeric transition), was found in natural radioactivity to be a result of the gamma decay of excited metastablenuclear isomers,which were in turn created from other types of decay. Although Alpha, beta, and gamma radiations were most commonly found, other types of emission were eventually discovered. Shortly after the discovery of thepositronin cosmic ray products, it was realized that the same process that operates in classical beta decay can also produce positrons (positron emission), along withneutrinos(classical beta decay produces antineutrinos).
Electron capture
[edit]In electron capture, some proton-rich nuclides were found to capture their own atomic electrons instead of emitting positrons, and subsequently, these nuclides emit only a neutrino and a gamma ray from the excited nucleus (and often alsoAuger electronsandcharacteristic X-rays,as a result of the re-ordering of electrons to fill the place of the missing captured electron). These types of decay involve the nuclear capture of electrons or emission of electrons or positrons, and thus acts to move a nucleus toward the ratio of neutrons to protons that has the least energy for a given total number ofnucleons.This consequently produces a more stable (lower energy) nucleus.
A hypothetical process of positron capture, analogous to electron capture, is theoretically possible in antimatter atoms, but has not been observed, as complex antimatter atoms beyondantiheliumare not experimentally available.[24]Such a decay would require antimatter atoms at least as complex asberyllium-7,which is the lightest known isotope of normal matter to undergo decay by electron capture.[25]
Nucleon emission
[edit]Shortly after the discovery of the neutron in 1932,Enrico Fermirealized that certain rare beta-decay reactions immediately yield neutrons as an additional decay particle, so called beta-delayedneutron emission.Neutron emission usually happens from nuclei that are in an excited state, such as the excited17O* produced from the beta decay of17N. The neutron emission process itself is controlled by thenuclear forceand therefore is extremely fast, sometimes referred to as "nearly instantaneous". Isolatedproton emissionwas eventually observed in some elements. It was also found that some heavy elements may undergospontaneous fissioninto products that vary in composition. In a phenomenon calledcluster decay,specific combinations of neutrons and protons other than Alpha particles (helium nuclei) were found to be spontaneously emitted from atoms.
More exotic types of decay
[edit]Other types of radioactive decay were found to emit previously seen particles but via different mechanisms. An example isinternal conversion,which results in an initial electron emission, and then often furthercharacteristic X-raysandAuger electronsemissions, although the internal conversion process involves neither beta nor gamma decay. A neutrino is not emitted, and none of the electron(s) and photon(s) emitted originate in the nucleus, even though the energy to emit all of them does originate there. Internal conversion decay, likeisomeric transitiongamma decay and neutron emission, involves the release of energy by an excited nuclide, without the transmutation of one element into another.
Rare events that involve a combination of two beta-decay-type events happening simultaneously are known (see below). Any decay process that does not violate the conservation of energy or momentum laws (and perhaps other particle conservation laws) is permitted to happen, although not all have been detected. An interesting example discussed in a final section, isbound state beta decayofrhenium-187.In this process, the beta electron-decay of the parent nuclide is not accompanied by beta electron emission, because the beta particle has been captured into the K-shell of the emitting atom. An antineutrino is emitted, as in all negative beta decays.
If energy circumstances are favorable, a given radionuclide may undergo many competing types of decay, with some atoms decaying by one route, and others decaying by another. An example iscopper-64,which has 29 protons, and 35 neutrons, which decays with a half-life of12.7004(13)hours.[26]This isotope has one unpaired proton and one unpaired neutron, so either the proton or the neutron can decay to the other particle, which has oppositeisospin.This particular nuclide (though not all nuclides in this situation) is more likely to decay throughbeta plus decay(61.52(26)%[26]) than throughelectron capture(38.48(26)%[26]). The excited energy states resulting from these decays which fail to end in a ground energy state, also produce later internal conversion andgamma decayin almost 0.5% of the time.
List of decay modes
[edit]
| Mode | Name | Action | Nucleus changes |
|---|---|---|---|
|
Alpha emission | AnAlpha particle(A= 4,Z= 2)emitted from nucleus | (A− 4,Z− 2) |
|
proton emission | Aprotonejected from nucleus | (A− 1,Z− 1) |
|
2-proton emission | Two protons ejected from nucleus simultaneously | (A− 2,Z− 2) |
|
neutron emission | Aneutronejected from nucleus | (A− 1,Z) |
|
2-neutron emission | Two neutrons ejected from nucleus simultaneously | (A− 2,Z) |
|
electron capture | A nucleus captures an orbiting electron and emits a neutrino; the daughter nucleus is left in an excited unstable state | (A,Z− 1) |
|
positron emission | A nuclear proton converts to a neutron by emitting a positron and an electron neutrino | (A,Z− 1) |
|
positron emission | In NUBASE2020, ß+refers to thecombinedrate of electron capture (ε) and positron emission (e+):ß+= ε + e+ | (A,Z− 1) |
|
β−decay | A nucleus emits anelectronand anelectron antineutrino | (A,Z+ 1) |
|
double β−decay | A nucleus emits two electrons and two antineutrinos | (A,Z+ 2) |
|
double β+decay | A nucleus emits two positrons and two neutrinos | (A,Z− 2) |
|
β−-delayed neutron emission | A nucleus decays by β−emission to an excited state, which then emits a neutron | (A− 1,Z+ 1) |
|
β−-delayed 2-neutron emission | A nucleus decays by β−emission to an excited state, which then emits two neutrons | (A− 2,Z+ 1) |
|
β−-delayed 3-neutron emission | A nucleus decays by β−emission to an excited state, which then emits three neutrons | (A− 3,Z+ 1) |
|
β+-delayed proton emission | A nucleus decays by β+emission to an excited state, which then emits a proton | (A− 1,Z− 2) |
|
β+-delayed 2-proton emission | A nucleus decays by β+emission to an excited state, which then emits two protons | (A− 2,Z− 3) |
|
β+-delayed 3-proton emission | A nucleus decays by β+emission to an excited state, which then emits three protons | (A− 3,Z− 4) |
|
β−-delayed Alpha emission | A nucleus decays by β−emission to an excited state, which then emits an α particle | (A− 4,Z− 1) |
|
β+-delayed Alpha emission | A nucleus decays by β+emission to an excited state, which then emits an a particle | (A− 4,Z− 3) |
|
β−-delayed deuteron emission | A nucleus decays by β−emission to an excited state, which then emits a deuteron | (A− 2,Z) |
|
β−-delayed triton emission | A nucleus decays by β−emission to an excited state, which then emits a triton | (A− 3,Z) |
|
cluster decay | A nucleus emits a specific type of smaller nucleus (A1,Z1) which is larger than an Alpha particle (e.g.14C,24Ne) | (A−A1,Z−Z1) &(A1,Z1) |
|
internal (isomeric) transition | A nucleus in a metastable state drops to a lower energy state by emitting a photon or ejecting an electron | (A,Z) |
|
spontaneous fission | A nucleus disintegrates into two or more smaller nuclei and other particles, all of which may vary with each decay | variable |
|
β+-delayed fission | A nucleus decays by β+emission to an excited state, which then undergoes spontaneous fission | β+ & variable |
|
β−-delayed fission | A nucleus decays by β−emission to an excited state, which then undergoes spontaneous fission | β−& variable |
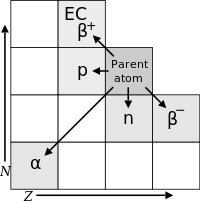
Decay chains and multiple modes
[edit]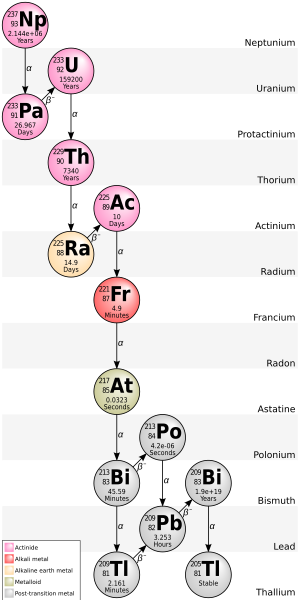
The daughter nuclide of a decay event may also be unstable (radioactive). In this case, it too will decay, producing radiation. The resulting second daughter nuclide may also be radioactive. This can lead to a sequence of several decay events called adecay chain(see this article for specific details of important natural decay chains). Eventually, a stable nuclide is produced. Any decay daughters that are the result of an Alpha decay will also result in helium atoms being created.
Some radionuclides may have several different paths of decay. For example,35.94(6)%[26]ofbismuth-212decays, through Alpha -emission, tothallium-208while64.06(6)%[26]ofbismuth-212decays, through beta-emission, topolonium-212.Boththallium-208andpolonium-212are radioactive daughter products of bismuth-212, and both decay directly to stablelead-208.
Occurrence and applications
[edit]According to theBig Bang theory,stable isotopes of the lightest three elements (H,He, and traces ofLi) were produced very shortly after the emergence of the universe, in a process calledBig Bang nucleosynthesis.These lightest stable nuclides (includingdeuterium) survive to today, but any radioactive isotopes of the light elements produced in the Big Bang (such astritium) have long since decayed. Isotopes of elements heavier than boron were not produced at all in the Big Bang, and these first five elements do not have any long-lived radioisotopes. Thus, all radioactive nuclei are, therefore, relatively young with respect to the birth of the universe, having formed later in various other types ofnucleosynthesisinstars(in particular,supernovae), and also during ongoing interactions between stable isotopes and energetic particles. For example,carbon-14,a radioactive nuclide with a half-life of only5700(30)years,[26]is constantly produced in Earth's upper atmosphere due to interactions between cosmic rays and nitrogen.
Nuclides that are produced by radioactive decay are calledradiogenic nuclides,whether they themselves arestableor not. There exist stable radiogenic nuclides that were formed from short-livedextinct radionuclidesin the early Solar System.[28][29]The extra presence of these stable radiogenic nuclides (such as xenon-129 from extinctiodine-129) against the background of primordialstable nuclidescan be inferred by various means.
Radioactive decay has been put to use in the technique ofradioisotopic labeling,which is used to track the passage of a chemical substance through a complex system (such as a livingorganism). A sample of the substance is synthesized with a high concentration of unstable atoms. The presence of the substance in one or another part of the system is determined by detecting the locations of decay events.
On the premise that radioactive decay is trulyrandom(rather than merelychaotic), it has been used inhardware random-number generators.Because the process is not thought to vary significantly in mechanism over time, it is also a valuable tool in estimating the absolute ages of certain materials. For geological materials, the radioisotopes and some of their decay products become trapped when a rock solidifies, and can then later be used (subject to many well-known qualifications) to estimate the date of the solidification. These include checking the results of several simultaneous processes and their products against each other, within the same sample. In a similar fashion, and also subject to qualification, the rate of formation of carbon-14 in various eras, the date of formation of organic matter within a certain period related to the isotope's half-life may be estimated, because the carbon-14 becomes trapped when the organic matter grows and incorporates the new carbon-14 from the air. Thereafter, the amount of carbon-14 in organic matter decreases according to decay processes that may also be independently cross-checked by other means (such as checking the carbon-14 in individual tree rings, for example).
Szilard–Chalmers effect
[edit]The Szilard–Chalmers effect is the breaking of a chemical bond as a result of a kinetic energy imparted from radioactive decay. It operates by the absorption of neutrons by an atom and subsequent emission of gamma rays, often with significant amounts of kinetic energy. This kinetic energy, byNewton's third law,pushes back on the decaying atom, which causes it to move with enough speed to break a chemical bond.[30]This effect can be used to separate isotopes by chemical means.
The Szilard–Chalmers effect was discovered in 1934 byLeó Szilárdand Thomas A. Chalmers.[31]They observed that after bombardment by neutrons, the breaking of a bond in liquid ethyl iodide allowed radioactive iodine to be removed.[32]
Origins of radioactive nuclides
[edit]Radioactiveprimordial nuclidesfound in theEarthare residues from ancientsupernovaexplosions that occurred before the formation of theSolar System.They are the fraction of radionuclides that survived from that time, through the formation of the primordial solarnebula,through planetaccretion,and up to the present time. The naturally occurring short-livedradiogenicradionuclides found in today'srocks,are the daughters of those radioactive primordial nuclides. Another minor source of naturally occurring radioactive nuclides arecosmogenic nuclides,that are formed by cosmic ray bombardment of material in the Earth'satmosphereorcrust.The decay of the radionuclides in rocks of the Earth'smantleandcrustcontribute significantly toEarth's internal heat budget.
Rates
[edit]Thedecay rate,oractivity,of a radioactive substance is characterized by the following time-independent parameters:
- Thehalf-life,t1/2,is the time taken for the activity of a given amount of aradioactive substanceto decay to half of its initial value.
- Thedecay constant,λ"lambda",the reciprocal of the mean lifetime (ins−1), sometimes referred to as simplydecay rate.
- Themean lifetime,τ"tau",the average lifetime (1/elife) of a radioactive particle before decay.
Although these are constants, they are associated with thestatistical behavior of populationsof atoms. In consequence, predictions using these constants are less accurate for minuscule samples of atoms.
In principle a half-life, a third-life, or even a (1/√2)-life, can be used in exactly the same way as half-life; but the mean life and half-lifet1/2have been adopted as standard times associated with exponential decay.
Those parameters can be related to the following time-dependent parameters:
- Total activity(or justactivity),A,is the number of decays per unit time of a radioactive sample.
- Number of particles,N,in the sample.
- Specific activity,a,is the number of decays per unit time per amount of substance of the sample at time set to zero (t= 0). "Amount of substance" can be the mass, volume or moles of the initial sample.
These are related as follows:
whereN0is the initial amount of active substance — substance that has the same percentage of unstable particles as when the substance was formed.
Mathematics
[edit]Universal law
[edit]The mathematics of radioactive decay depend on a key assumption that a nucleus of a radionuclide has no "memory" or way of translating its history into its present behavior. A nucleus does not "age" with the passage of time. Thus, the probability of its breaking down does not increase with time but stays constant, no matter how long the nucleus has existed. This constant probability may differ greatly between one type of nucleus and another, leading to the many different observed decay rates. However, whatever the probability is, it does not change over time. This is in marked contrast to complex objects that do show aging, such as automobiles and humans. These aging systems do have a chance of breakdown per unit of time that increases from the moment they begin their existence.
Aggregate processes, like the radioactive decay of a lump of atoms, for which the single-event probability of realization is very small but in which the number of time-slices is so large that there is nevertheless a reasonable rate of events, are modelled by thePoisson distribution,which is discrete. Radioactive decay andnuclear particle reactionsare two examples of such aggregate processes.[33]The mathematics of Poisson processes reduce to the law ofexponential decay,which describes the statistical behaviour of a large number of nuclei, rather than one individual nucleus. In the following formalism, the number of nuclei or the nuclei populationN,is of course a discrete variable (anatural number)—but for any physical sampleNis so large that it can be treated as a continuous variable.Differential calculusis used to model the behaviour of nuclear decay.
One-decay process
[edit]Consider the case of a nuclideAthat decays into anotherBby some processA→B(emission of other particles, likeelectron neutrinos
ν
eandelectronse−as inbeta decay,are irrelevant in what follows). The decay of an unstable nucleus is entirely random in time so it is impossible to predict when a particular atom will decay. However, it is equally likely to decay at any instant in time. Therefore, given a sample of a particular radioisotope, the number of decay events−dNexpected to occur in a small interval of timedtis proportional to the number of atoms presentN,that is[34]
Particular radionuclides decay at different rates, so each has its own decay constantλ.The expected decay−dN/Nis proportional to an increment of time,dt:
The negative sign indicates thatNdecreases as time increases, as the decay events follow one after another. The solution to this first-orderdifferential equationis thefunction:
whereN0is the value ofNat timet= 0, with the decay constant expressed asλ[34]
We have for all timet:
whereNtotalis the constant number of particles throughout the decay process, which is equal to the initial number ofAnuclides since this is the initial substance.
If the number of non-decayedAnuclei is:
then the number of nuclei ofB(i.e. the number of decayedAnuclei) is
The number of decays observed over a given interval obeysPoisson statistics.If the average number of decays is⟨N⟩,the probability of a given number of decaysNis[34]
Chain-decay processes
[edit]Chain of two decays
[edit]Now consider the case of a chain of two decays: one nuclideAdecaying into anotherBby one process, thenBdecaying into anotherCby a second process, i.e.A → B → C.The previous equation cannot be applied to the decay chain, but can be generalized as follows. SinceAdecays intoB,thenBdecays intoC,the activity ofAadds to the total number ofBnuclides in the present sample,beforethoseBnuclides decay and reduce the number of nuclides leading to the later sample. In other words, the number of second generation nucleiBincreases as a result of the first generation nuclei decay ofA,and decreases as a result of its own decay into the third generation nucleiC.[35]The sum of these two terms gives the law for a decay chain for two nuclides:
The rate of change ofNB,that isdNB/dt,is related to the changes in the amounts ofAandB,NBcan increase asBis produced fromAand decrease asBproducesC.
Re-writing using the previous results:
The subscripts simply refer to the respective nuclides, i.e.NAis the number of nuclides of typeA;NA0is the initial number of nuclides of typeA;λAis the decay constant forA– and similarly for nuclideB.Solving this equation forNBgives:
In the case whereBis a stable nuclide (λB= 0), this equation reduces to the previous solution:
as shown above for one decay. The solution can be found by theintegration factormethod, where the integrating factor iseλBt.This case is perhaps the most useful since it can derive both the one-decay equation (above) and the equation for multi-decay chains (below) more directly.
Chain of any number of decays
[edit]For the general case of any number of consecutive decays in a decay chain, i.e.A1→ A2··· → Ai··· → AD,whereDis the number of decays andiis a dummy index (i= 1, 2, 3,...,D), each nuclide population can be found in terms of the previous population. In this caseN2= 0,N3= 0,...,ND= 0.Using the above result in a recursive form:
The general solution to the recursive problem is given byBateman's equations:[36]
Alternative modes
[edit]In all of the above examples, the initial nuclide decays into just one product.[37]Consider the case of one initial nuclide that can decay into either of two products, that isA → BandA → Cin parallel. For example, in a sample ofpotassium-40,89.3% of the nuclei decay tocalcium-40and 10.7% toargon-40.We have for all timet:
which is constant, since the total number of nuclides remains constant. Differentiating with respect to time:
defining thetotal decay constantλin terms of the sum ofpartial decay constantsλBandλC:
Solving this equation forNA:
whereNA0is the initial number of nuclide A. When measuring the production of one nuclide, one can only observe the total decay constantλ.The decay constantsλBandλCdetermine the probability for the decay to result in productsBorCas follows:
because the fractionλB/λof nuclei decay intoBwhile the fractionλC/λof nuclei decay intoC.
Corollaries of laws
[edit]The above equations can also be written using quantities related to the number of nuclide particlesNin a sample;
- The activity:A=λN.
- Theamount of substance:n=N/NA.
- Themass:m=Mn=MN/NA.
whereNA=6.02214076×1023mol−1[38]is theAvogadro constant,Mis themolar massof the substance in kg/mol, and the amount of the substancenis inmoles.
Decay timing: definitions and relations
[edit]Time constant and mean-life
[edit]For the one-decay solutionA → B:
the equation indicates that the decay constantλhas units oft−1,and can thus also be represented as 1/τ,whereτis a characteristic time of the process called thetime constant.
In a radioactive decay process, this time constant is also themean lifetimefor decaying atoms. Each atom "lives" for a finite amount of time before it decays, and it may be shown that this mean lifetime is thearithmetic meanof all the atoms' lifetimes, and that it isτ,which again is related to the decay constant as follows:
This form is also true for two-decay processes simultaneouslyA → B + C,inserting the equivalent values of decay constants (as given above)
into the decay solution leads to:

Half-life
[edit]A more commonly used parameter is the half-lifeT1/2.Given a sample of a particular radionuclide, the half-life is the time taken for half the radionuclide's atoms to decay. For the case of one-decay nuclear reactions:
the half-life is related to the decay constant as follows: setN =N0/2andt=T1/2to obtain
This relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent, while those that radiate weakly endure longer.Half-lives of known radionuclidesvary by almost 54 orders of magnitude, from more than2.25(9)×1024years (6.9×1031sec) for the very nearly stable nuclide128Te,to8.6(6)×10−23seconds for the highly unstable nuclide5H.[26]
The factor ofln(2)in the above relations results from the fact that the concept of "half-life" is merely a way of selecting a different base other than the natural baseefor the lifetime expression. The time constantτis thee−1-life, the time until only 1/eremains, about 36.8%, rather than the 50% in the half-life of a radionuclide. Thus,τis longer thant1/2.The following equation can be shown to be valid:
Since radioactive decay is exponential with a constant probability, each process could as easily be described with a different constant time period that (for example) gave its "(1/3)-life" (how long until only 1/3 is left) or "(1/10)-life" (a time period until only 10% is left), and so on. Thus, the choice ofτandt1/2for marker-times, are only for convenience, and from convention. They reflect a fundamental principle only in so much as they show that thesame proportionof a given radioactive substance will decay, during any time-period that one chooses.
Mathematically, thenthlife for the above situation would be found in the same way as above—by settingN = N0/n,t=T1/nand substituting into the decay solution to obtain
Example for carbon-14
[edit]Carbon-14has a half-life of5700(30)years[26]and a decay rate of 14 disintegrations per minute (dpm) per gram of natural carbon.
If an artifact is found to have radioactivity of 4 dpm per gram of its present C, we can find the approximate age of the object using the above equation:
where:
Changing rates
[edit]The radioactive decay modes of electron capture and internal conversion are known to be slightly sensitive to chemical and environmental effects that change the electronic structure of the atom, which in turn affects the presence of1sand2selectrons that participate in the decay process. A small number of nuclides are affected.[39]For example,chemical bondscan affect the rate of electron capture to a small degree (in general, less than 1%) depending on the proximity of electrons to the nucleus. In7Be, a difference of 0.9% has been observed between half-lives in metallic and insulating environments.[40]This relatively large effect is because beryllium is a small atom whose valence electrons are in2satomic orbitals,which are subject to electron capture in7Be because (like allsatomic orbitals in all atoms) they naturally penetrate into the nucleus.
In 1992, Jung et al. of the Darmstadt Heavy-Ion Research group observed an accelerated β−decay of163Dy66+.Although neutral163Dy is a stable isotope, the fully ionized163Dy66+undergoes β−decayinto the K and L shellsto163Ho66+with a half-life of 47 days.[41]
Rhenium-187is another spectacular example.187Re normally undergoes beta decay to187Os with a half-life of 41.6 × 109years,[42]but studies using fully ionised187Reatoms (bare nuclei) have found that this can decrease to only 32.9 years.[43]This is attributed to "bound-state β−decay"of the fully ionised atom – the electron is emitted into the" K-shell "(1satomic orbital), which cannot occur for neutral atoms in which all low-lying bound states are occupied.[44]

A number of experiments have found that decay rates of other modes of artificial and naturally occurring radioisotopes are, to a high degree of precision, unaffected by external conditions such as temperature, pressure, the chemical environment, and electric, magnetic, or gravitational fields.[45]Comparison of laboratory experiments over the last century, studies of the Oklonatural nuclear reactor(which exemplified the effects of thermal neutrons on nuclear decay), and astrophysical observations of the luminosity decays of distant supernovae (which occurred far away so the light has taken a great deal of time to reach us), for example, strongly indicate that unperturbed decay rates have been constant (at least to within the limitations of small experimental errors) as a function of time as well.[citation needed]
Recent results suggest the possibility that decay rates might have a weak dependence on environmental factors. It has been suggested that measurements of decay rates ofsilicon-32,manganese-54,andradium-226exhibit small seasonal variations (of the order of 0.1%).[46][47][48]However, such measurements are highly susceptible to systematic errors, and a subsequent paper[49]has found no evidence for such correlations in seven other isotopes (22Na,44Ti,108Ag,121Sn,133Ba,241Am,238Pu), and sets upper limits on the size of any such effects. The decay ofradon-222was once reported to exhibit large 4% peak-to-peak seasonal variations (see plot),[50]which were proposed to be related to eithersolar flareactivity or the distance from the Sun, but detailed analysis of the experiment's design flaws, along with comparisons to other, much more stringent and systematically controlled, experiments refute this claim.[51]
GSI anomaly
[edit]An unexpected series of experimental results for the rate of decay of heavyhighly chargedradioactiveionscirculating in astorage ringhas provoked theoretical activity in an effort to find a convincing explanation. The rates ofweakdecay of two radioactive species with half lives of about 40 s and 200 s are found to have a significantoscillatorymodulation,with a period of about 7 s.[52] The observed phenomenon is known as theGSI anomaly,as the storage ring is a facility at theGSI Helmholtz Centre for Heavy Ion ResearchinDarmstadt,Germany.As the decay process produces anelectron neutrino,some of the proposed explanations for the observed rate oscillation invoke neutrino properties. Initial ideas related toflavour oscillationmet with skepticism.[53]A more recent proposal involves mass differences between neutrino masseigenstates.[54]
Theoretical basis
[edit]The neutrons and protons that constitute nuclei, as well as other particles that approach close enough to them, are governed by several interactions. The nuclear force (also known asresidualstrong force), not observed at the familiarmacroscopicscale, is the most powerful force over subatomic distances. Theelectrostatic forceis almost always significant, and, in the case ofbeta decay,theweak nuclear forceis also involved.
The combined effects of these forces produces a number of different phenomena in which energy may be released by rearrangement of particles in the nucleus, or else the change of one type of particle into others. These rearrangements and transformations may be hindered energetically so that they do not occur immediately. Radioactive decay half-life of nuclides has been measured over timescales of 54 orders of magnitude, from8.6(6)×10−23seconds (forhydrogen-5) to7.10(28)×1031seconds (fortellurium-128).[26]The limits of these timescales are set by the sensitivity of instrumentation only, and there are no known natural limits to how brief[citation needed]or long a decay half-life for radioactive decay of a radionuclide may be. A radioactive nucleus (or any excited system in quantum mechanics) is unstable, and can, thus,spontaneouslystabilize to a less-excited system. The resulting transformation alters the structure of the nucleus and results in the emission of either a photon or a high-velocity particle that has mass (such as an electron, Alpha particle, or other type).[55]
Hazard warning signs
[edit]-
The trefoil symbol used to warn of presence of radioactive material or ionising radiation
-
2007 ISO radioactivityhazard symbolintended for IAEA Category 1, 2 and 3 sources defined as dangerous sources capable of death or serious injury[56]
-
One of several dangerous goods transport classification signs for radioactive materials
See also
[edit]- Actinides in the environment
- Background radiation
- Chernobyl disaster
- Crimes involving radioactive substances
- Decay chain
- Decay correction
- Fallout shelter
- Geiger counter
- Induced radioactivity
- Lists of nuclear disasters and radioactive incidents
- National Council on Radiation Protection and Measurements
- Nuclear engineering
- Nuclear pharmacy
- Nuclear physics
- Nuclear power
- Nuclear chain reaction
- Particle decay
- Poisson process
- Radiation therapy
- Radioactive contamination
- Radioactivity in biology
- Radiometric dating
- Stochastic
- Transient equilibrium
![]() Nuclear technology portal
Nuclear technology portal
![]() Physics portal
Physics portal
Notes
[edit]- ^Radionuclide is the more correct term, but radioisotope is also used. The difference between isotope and nuclide is explained atIsotope § Isotope vs. nuclide.
References
[edit]Inline
[edit]- ^"Radioactivity: Weak Forces".Radioactivity.EDP Sciences. Archived fromthe originalon 12 August 2021.Retrieved4 March2020.
- ^Stabin, Michael G. (2007)."3".In Stabin, Michael G (ed.).Radiation Protection and Dosimetry: An Introduction to Health Physics.Springer.doi:10.1007/978-0-387-49983-3.ISBN978-0-387-49982-6.
- ^Best, Lara; Rodrigues, George; Velker, Vikram (2013). "1.3".Radiation Oncology Primer and Review.Demos Medical Publishing.ISBN978-1-62070-004-4.
- ^Loveland, W.; Morrissey, D.;Seaborg, G.T.(2006).Modern Nuclear Chemistry.Wiley-Interscience. p. 57.Bibcode:2005mnc..book.....L.ISBN978-0-471-11532-8.
- ^Mould, Richard F. (1995).A century of X-rays and radioactivity in medicine: with emphasis on photographic records of the early years(Reprint. with minor corr ed.). Bristol: Inst. of Physics Publ. p. 12.ISBN978-0-7503-0224-1.
- ^Henri Becquerel (1896)."Sur les radiations émises par phosphorescence".Comptes Rendus.122:420–421.
- ^Comptes Rendus122:420 (1896),translated by Carmen Giunta.Retrieved 12 April 2021.
- ^Henri Becquerel (1896)."Sur les radiations invisibles émises par les corps phosphorescents".Comptes Rendus.122:501–503.
- ^Comptes Rendus122:501–503 (1896),translated by Carmen Giunta.Retrieved 12 April 2021.
- ^Kasimir Fajans, "Radioactive transformations and the periodic system of the elements".Berichte der Deutschen Chemischen Gesellschaft,Nr. 46, 1913, pp. 422–439
- ^Frederick Soddy, "The Radio Elements and the Periodic Law", Chem. News, Nr. 107, 1913, pp. 97–99
- ^abL'Annunziata, Michael F. (2007).Radioactivity: Introduction and History.Amsterdam, Netherlands: Elsevier Science. p. 2.ISBN9780080548883.
- ^Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002).General chemistry(8th ed.). Prentice Hall. p. 1025.ISBN0-13-014329-4.
- ^Sansare, K.; Khanna, V.; Karjodkar, F. (2011)."Early victims of X-rays: a tribute and current perception".Dentomaxillofacial Radiology.40(2): 123–125.doi:10.1259/dmfr/73488299.ISSN0250-832X.PMC3520298.PMID21239576.
- ^ab"Ronald L. Kathern and Paul L. Ziemer, he First Fifty Years of Radiation Protection, physics.isu.edu".Archived fromthe originalon 12 September 2017.Retrieved25 November2013.
- ^Hrabak, M.; Padovan, R.S.; Kralik, M.; Ozretic, D.; Potocki, K. (July 2008)."Nikola Tesla and the Discovery of X-rays".RadioGraphics.28(4): 1189–92.doi:10.1148/rg.284075206.PMID18635636.
- ^Rentetzi, Maria (7 November 2017)."Marie Curie and the perils in radium".Physics Today(11): 30676.Bibcode:2017PhT..2017k0676R.doi:10.1063/PT.6.4.20171107a.Retrieved3 May2022.
- ^Clarke, R.H.; J. Valentin (2009)."The History of ICRP and the Evolution of its Policies"(PDF).Annals of the ICRP.ICRP Publication 109.39(1): 75–110.doi:10.1016/j.icrp.2009.07.009.S2CID71278114.Retrieved12 May2012.
- ^Daniels, M.; et al. (2020)."Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis".J Natl Cancer Inst Monogr.56(July 1): 188–200.doi:10.1093/jncimonographs/lgaa010.ISSN1434-6001.PMC8454205.PMID32657347.
- ^Venturi, Sebastiano (January 2021)."Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage – Scientific, Environmental, Geopolitical, and Economic Aspects".International Journal of Environmental Research and Public Health.18(17): 8934.doi:10.3390/ijerph18178934.PMC8431133.PMID34501532.
 Text was copied from this source, which is available under aCreative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under aCreative Commons Attribution 4.0 International License.
- ^Rutherford, Ernest (6 October 1910)."Radium Standards and Nomenclature".Nature.84(2136): 430–431.Bibcode:1910Natur..84..430R.doi:10.1038/084430a0.
- ^10 CFR 20.1005.US Nuclear Regulatory Commission. 2009.
- ^The Council of the European Communities (21 December 1979)."Council Directive 80/181/EEC of 20 December 1979 on the approximation of the laws of the Member States relating to Unit of measurement and on the repeal of Directive 71/354/EEC".Retrieved19 May2012.
- ^"Radioactive Decay".chemed.chem.purdue.edu.Retrieved5 May2022.
- ^"CH103 – Chapter 3: Radioactivity and Nuclear Chemistry – Chemistry".Retrieved5 July2022.
- ^abcdefghiKondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (March 2021)."The NUBASE2020 evaluation of nuclear physics properties \ast".Chinese Physics C.45(3): 030001.doi:10.1088/1674-1137/abddae.ISSN1674-1137.S2CID233794940.
- ^Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021)."The NUBASE2020 evaluation of nuclear properties"(PDF).Chinese Physics C.45(3): 030001.doi:10.1088/1674-1137/abddae.
- ^Clayton, Donald D. (1983).Principles of Stellar Evolution and Nucleosynthesis(2nd ed.). University of Chicago Press. p.75.ISBN978-0-226-10953-4.
- ^Bolt, B.A.; Packard, R.E.; Price, P.B. (2007)."John H. Reynolds, Physics: Berkeley".The University of California, Berkeley.Retrieved1 October2007.
- ^"Szilard-Chalmers effect - Oxford Reference".oxfordreference.Retrieved27 December2019.
- ^Szilard, Leó; Chalmers, Thomas A. (1934)."Chemical separation of the radioactive element from its bombarded isotope in the Fermi effect".Nature.134(3386): 462.Bibcode:1934Natur.134..462S.doi:10.1038/134462b0.S2CID4129460.
- ^Harbottle, Garman; Sutin, Norman (1 January 1959), Emeléus, H. J.; Sharpe, A. G. (eds.),The Szilard-Chalmers Reaction in Solids,Advances in Inorganic Chemistry and Radiochemistry, vol. 1, Academic Press, pp. 267–314,doi:10.1016/S0065-2792(08)60256-3,ISBN9780120236015,retrieved19 March2020
- ^Leo, William R. (1992). "Ch. 4".Statistics and the treatment of experimental data(Techniques for Nuclear and Particle Physics Experiments ed.). Springer-Verlag.
- ^abcPatel, S.B. (2000).Nuclear physics: an introduction.New Delhi: New Age International. pp. 62–72.ISBN978-81-224-0125-7.
- ^Introductory Nuclear Physics, K.S. Krane, 1988, John Wiley & Sons Inc,ISBN978-0-471-80553-3
- ^Cetnar, Jerzy (May 2006). "General solution of Bateman equations for nuclear transmutations".Annals of Nuclear Energy.33(7): 640–645.Bibcode:2006AnNuE..33..640C.doi:10.1016/j.anucene.2006.02.004.
- ^K.S. Krane (1988).Introductory Nuclear Physics.John Wiley & Sons Inc. p. 164.ISBN978-0-471-80553-3.
- ^"2022 CODATA Value: Avogadro constant".The NIST Reference on Constants, Units, and Uncertainty.NIST.May 2024.Retrieved18 May2024.
- ^Emery, G T (December 1972)."Perturbation of Nuclear Decay Rates".Annual Review of Nuclear Science.22(1): 165–202.Bibcode:1972ARNPS..22..165E.doi:10.1146/annurev.ns.22.120172.001121.ISSN0066-4243.Retrieved23 February2022.
- ^Wang, B.; et al. (2006). "Change of the 7Be electron capture half-life in metallic environments".The European Physical Journal A.28(3): 375–377.Bibcode:2006EPJA...28..375W.doi:10.1140/epja/i2006-10068-x.ISSN1434-6001.S2CID121883028.
- ^Jung, M.; et al. (1992). "First observation of bound-state β−decay ".Physical Review Letters.69(15): 2164–2167.Bibcode:1992PhRvL..69.2164J.doi:10.1103/PhysRevLett.69.2164.ISSN0031-9007.PMID10046415.
- ^Smoliar, M.I.; Walker, R.J.; Morgan, J.W. (1996). "Re-Os ages of group IIA, IIIA, IVA, and IVB iron meteorites".Science.271(5252): 1099–1102.Bibcode:1996Sci...271.1099S.doi:10.1126/science.271.5252.1099.S2CID96376008.
- ^ Bosch, F.; et al. (1996). "Observation of bound-state beta minus decay of fully ionized187Re:187Re–187Os Cosmochronometry ".Physical Review Letters.77(26): 5190–5193.Bibcode:1996PhRvL..77.5190B.doi:10.1103/PhysRevLett.77.5190.PMID10062738.
- ^Bosch, F.; et al. (1996). "Observation of bound-state β– decay of fully ionized187Re:187Re-187Os Cosmochronometry ".Physical Review Letters.77(26): 5190–5193.Bibcode:1996PhRvL..77.5190B.doi:10.1103/PhysRevLett.77.5190.PMID10062738.
- ^Emery, G.T. (1972)."Perturbation of Nuclear Decay Rates".Annual Review of Nuclear Science.22(1): 165–202.Bibcode:1972ARNPS..22..165E.doi:10.1146/annurev.ns.22.120172.001121.
- ^"The mystery of varying nuclear decay".Physics World.2 October 2008.
- ^Jenkins, Jere H.; Fischbach, Ephraim (2009). "Perturbation of Nuclear Decay Rates During the Solar Flare of 13 December 2006".Astroparticle Physics.31(6): 407–411.arXiv:0808.3156.Bibcode:2009APh....31..407J.doi:10.1016/j.astropartphys.2009.04.005.S2CID118863334.
- ^Jenkins, J.H.; Fischbach, Ephraim; Buncher, John B.; Gruenwald, John T.; Krause, Dennis E.; Mattes, Joshua J. (2009). "Evidence of correlations between nuclear decay rates and Earth–Sun distance".Astroparticle Physics.32(1): 42–46.arXiv:0808.3283.Bibcode:2009APh....32...42J.doi:10.1016/j.astropartphys.2009.05.004.S2CID119113836.
- ^Norman, E.B.; Browne, Edgardo; Shugart, Howard A.; Joshi, Tenzing H.; Firestone, Richard B. (2009)."Evidence against correlations between nuclear decay rates and Earth–Sun distance"(PDF).Astroparticle Physics.31(2): 135–137.arXiv:0810.3265.Bibcode:2009APh....31..135N.doi:10.1016/j.astropartphys.2008.12.004.S2CID7051382.Archived fromthe original(PDF)on 29 June 2010.Retrieved23 September2009.
- ^Sturrock, P.A.; Steinitz, G.; Fischbach, E.; Javorsek, D.; Jenkins, J.H. (2012). "Analysis of gamma radiation from a radon source: Indications of a solar influence".Astroparticle Physics.36(1): 18–25.arXiv:1205.0205.Bibcode:2012APh....36...18S.doi:10.1016/j.astropartphys.2012.04.009.ISSN0927-6505.S2CID119163371.
- ^Pommé, S.; Lutter, G.; Marouli, M.; Kossert, K.; Nähle, O. (1 January 2018)."On the claim of modulations in radon decay and their association with solar rotation".Astroparticle Physics.97:38–45.Bibcode:2018APh....97...38P.doi:10.1016/j.astropartphys.2017.10.011.ISSN0927-6505.
- ^Kienle P, Bosch F, Bühler P, Faestermanna T, Litvinov Yu.A., Winckler N, et al. (2013). "High-resolution measurement of the time-modulated orbital electron capture and of the β+decay of hydrogen-like142Pm60+ions ".Physics Letters B.726(4–5): 638–645.arXiv:1309.7294.Bibcode:2013PhLB..726..638K.doi:10.1016/j.physletb.2013.09.033.ISSN0370-2693.S2CID55085840.
- ^Giunti, Carlo (2009). "The GSI Time Anomaly: Facts and Fiction".Nuclear Physics B: Proceedings Supplements.188:43–45.arXiv:0812.1887.Bibcode:2009NuPhS.188...43G.doi:10.1016/j.nuclphysbps.2009.02.009.ISSN0920-5632.S2CID10196271.
- ^Gal, Avraham (2016)."Neutrino Signals in Electron-Capture Storage-Ring Experiments".Symmetry.8(6): 49.arXiv:1407.1789.Bibcode:2016Symm....8...49G.doi:10.3390/sym8060049.ISSN2073-8994.S2CID14287612.
- ^Noboru Takigawa and Kouhei Washiyama (2017)Fundamentals of Nuclear PhysicsSpringer
- ^IAEA news release Feb 2007
General
[edit]- "Radioactivity",Encyclopædia Britannica. 2006.Encyclopædia Britannica Online.December 18, 2006
- Radio-activity by Ernest Rutherford Phd,Encyclopædia Britannica Eleventh Edition
External links
[edit]- The Lund/LBNL Nuclear Data Search– Contains tabulated information on radioactive decay types and energies.
- Nomenclature of nuclear chemistry
- Specific activity and related topics.
- The Live Chart of Nuclides – IAEA
- Interactive Chart of NuclidesArchived10 October 2018 at theWayback Machine
- Health Physics Society Public Education Website
- Beach, Chandler B., ed. (1914)...Chicago: F. E. Compton and Co.
- Annotated bibliography for radioactivity from the Alsos Digital Library for Nuclear IssuesArchived7 October 2010 at theWayback Machine
- Stochastic Java applet on the decay of radioactive atomsArchived13 August 2011 at theWayback Machineby Wolfgang Bauer
- Stochastic Flash simulation on the decay of radioactive atomsby David M. Harrison
- "Henri Becquerel: The Discovery of Radioactivity", Becquerel's 1896 articles online and analyzed onBibNum[click 'à télécharger' for English version].
- "Radioactive change", Rutherford & Soddy article (1903), online and analyzed onBibnum[click 'à télécharger' for English version]
- ^Pfeifer, Kent B; Weber, Thomas M; Martin, James E (29 November 2023)."Development of local-power-free, remoteα-particle detection using optical fibers ".Journal of Radiation Research.65(1): 136–143.doi:10.1093/jrr/rrad092.ISSN0449-3060.PMC10803159.PMID38037422.
- ^Masoodian, Seyed Mohammad; Toolabi, Karamollah; Omidifar, Abolfazl; Zabihi, Hossein; Rahimipour, Ali; Shanaki, Mehrnoosh (1 May 2020)."Increased mRNA Expression of CTRP3 and CTRP9 in Adipose Tissue from Obese Women: Is it Linked to Obesity-Related Parameters and mRNA Expression of Inflammatory Cytokines?".Reports of Biochemistry and Molecular Biology.9(1): 71–81.doi:10.29252/rbmb.9.1.71.ISSN2322-3480.PMC7424416.PMID32821754.

![{\displaystyle {\begin{aligned}t_{1/2}&={\frac {\ln(2)}{\lambda }}=\tau \ln(2)\\[2pt]A&=-{\frac {\mathrm {d} N}{\mathrm {d} t}}=\lambda N={\frac {\ln(2)}{t_{1/2}}}N\\[2pt]S_{A}a_{0}&=-{\frac {\mathrm {d} N}{\mathrm {d} t}}{\bigg |}_{t=0}=\lambda N_{0}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/08fd79adb91a3896db0855d30c72096055431cbf)










![{\displaystyle \lim _{\lambda _{B}\rightarrow 0}\left[{\frac {N_{A0}\lambda _{A}}{\lambda _{B}-\lambda _{A}}}\left(e^{-\lambda _{A}t}-e^{-\lambda _{B}t}\right)\right]={\frac {N_{A0}\lambda _{A}}{0-\lambda _{A}}}\left(e^{-\lambda _{A}t}-1\right)=N_{A0}\left(1-e^{-\lambda _{A}t}\right),}](https://wikimedia.org/api/rest_v1/media/math/render/svg/982ae50245eea1305c63a7b97be54ea1e2a19ccf)

![{\displaystyle {\begin{aligned}N_{D}&={\frac {N_{1}(0)}{\lambda _{D}}}\sum _{i=1}^{D}\lambda _{i}c_{i}e^{-\lambda _{i}t}\\[3pt]c_{i}&=\prod _{j=1,i\neq j}^{D}{\frac {\lambda _{j}}{\lambda _{j}-\lambda _{i}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4cd85cd77b00707ebf272a4cb25d5b2768e2ab39)
















![2007 ISO radioactivity hazard symbol intended for IAEA Category 1, 2 and 3 sources defined as dangerous sources capable of death or serious injury[56]](https://upload.wikimedia.org/wikipedia/commons/thumb/3/35/Logo_iso_radiation.svg/120px-Logo_iso_radiation.svg.png)
