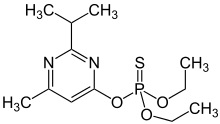
Diazinon
This articleneeds additional citations forverification.(January 2013) |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
O,O-DiethylO-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate | |
| Other names
Diethoxy-[(2-isopropyl-6-methyl-4-pyrimidinyl)oxy]-thioxophosphorane
Basudin Diazide Spectracide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.795 |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C12H21N2O3PS | |
| Molar mass | 304.34g·mol−1 |
| Appearance | Colorless to dark brown liquid |
| Odor | faint,ester-like |
| Density | 1.116-1.118 g/cm3at 20 °C[1] |
| Boiling point | decomposes[2] |
| 40 mg/L[3] | |
| logP | 3.81 (octanol/water)[4] |
| Pharmacology | |
| QP53AF03(WHO) | |
| Hazards | |
| Flash point | 82 °C; 180 °F; 355 K[2] |
| NIOSH(US health exposure limits): | |
PEL(Permissible)
|
none[2] |
REL(Recommended)
|
TWA 0.1 mg/m3[skin][2] |
IDLH(Immediate danger)
|
N.D.[2] |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Diazinon(IUPACname:O,O-DiethylO-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate,INN-Dimpylate), a colorless to dark brown liquid, is a thiophosphoric acid ester developed in 1952 byCiba-Geigy,a Swiss chemical company (laterNovartisand thenSyngenta). It is a nonsystemicorganophosphateinsecticideformerly used to controlcockroaches,silverfish,ants,andfleasin residential, non-food buildings. Diazinon was heavily used during the 1970s and early 1980s for general-purpose gardening use and indoor pest control. A bait form was used to control scavengerwaspsin the western U.S. Diazinon is used in flea collars for domestic pets in Australia and New Zealand. Diazinon is a major component in the "Golden Fleece" brandsheep dip.Residential uses of diazinon were outlawed in the U.S. in 2004 because of human health risks[5]but it is still approved for agricultural uses. An emergency antidote isatropine.[6]
Specific Health Risks:Routes of contact for Diazinon include breathing it, consuming it, or contact with skin. The negative health effects when in contact with Diazinon includes eye watering, drool or runny nose, not having any appetite, throwing up, intense coughing, abdominal pain, or even stiffness in various muscles/paralysis.[7]Headaches can also be health risks.[8]Some other physiological effects include pinpoint pupils, increased heart rate, seizures, or even coma. Larger exposure to diazinon is known to yield these risks. If it is a smaller exposure, the symptoms may be reduced such as just a runny nose or some eye watering.[9]One thing to note is that while Diazinon has many different health risks, the EPA has not categorized it as a carcinogen.[10]
History
[edit]Diazinon was developed in 1952 by the Swiss companyCiba-Geigy(nowNovartis) to replace the formerly dominantinsecticideDDT.In 1939, the chemistPaul Hermann Müllerfrom the then-independent Geigy company had discovered that DDT was effective againstmalaria-bearing insects. This capability made use of DDT important enough that Müller even received the 1948Nobel Prize in Medicine.[citation needed]
However, as the decades following the award passed, DDT was found to be such an environmental danger that developed countries and eventually world-level organizations banned the insecticide for all purposes except for combatingdisease-vectorinsects, leading Ciba-Geigy to research alternatives.[citation needed]
Diazinon became available for mass use in 1955, while DDT production tapered. Before 1970, diazinon had issues withcontaminantsin itssolution;but by the 1970s, alternativepurification methodswere used to reduce the residual, unwanted materials.[citation needed]
After this processing improvement, diazinon became an all-purpose, indoor-and-outdoor, commercial pest control product. In 2004, the US outlawed residential use of diazinon when the EPA determined that its ability to damage the nervous system posed a risk to human health (especially the health of children).[5]The chemical is still used for industrial agricultural purposes.
Synthesis
[edit]According to the German Patent bureau, the industrial synthesis of diazinon is as follows:
- β-isobutyrylaminocrotonic acid amine was cyclized with NaOR (R is either a hydrogen or aliphatic chain of 1 to 8 carbons) in a mixture of 0 to 100% by weight of water and an alcohol having 1 to 8 carbon atoms, above 90°C (but below the boiling point of the mixture used). Sodium pyrimidinolate was precipitated out in an inert solvent, such as benzene, with simultaneous removal of the water formed. The potassium salt is then reacted with diethylthiophosphoryl chloride by heating for several hours. When the reaction finished, the potassium chloride formed was washed with water and the solvent was removed under reduced pressure leaving diazinon.[citation needed]
Metabolism and mechanism of action
[edit]Diazinon functions as anacetylcholinesterase (AChE) inhibitor.This enzyme breaks down the neurotransmitteracetylcholine(ACh) intocholineand anacetategroup.[11]The inhibition of AChE causes an abnormal accumulation of ACh in thesynaptic cleft.[citation needed]
When diazinon enters the body, it is oxidatively decomposed to diazoxon, anorganophosphatecompound that is much more poisonous than diazinon; it mainly causes the inhibition of AChE.[12]The conversion of diazinon to diazoxon (Reaction 1) is performed by thelivermicrosomalenzyme system and requires O2andNADPH.Diazinon can also be decomposed via oxidation in the liver (Reaction 2). Both reactions are possible, and likely are catalyzed nonspecifically by the same mixed functionoxidase.Diazoxon is further broken down byhydrolasesin the microsomal and other subcellular functions within the liver (Reaction 3). Mammals metabolize diazoxon with a half-life of 2 to 6 weeks. Insects lack this hydrolysis step, which allows the toxic substance to accumulate rapidly; the detoxification of diazoxon is processed through the microsomal mixed function oxidase system. Although not fully understood, it is believed that this is the cause for the selectivity of diazinon against insects. After the hydrolysis or oxidation diazinon is broken down further (Reaction 4).[citation needed]

Removal of diazinon
[edit]To date, several methods such as electrochemistry, adsorption, enzymatic biodegradation, and photocatalysis have been tested for the elimination of diazinon from aqueous solutions. The removal oforganophosphates(OPE) from water by adsorption techniques is regarded as one of the competitive methods because of its simple operation and low cost. Development of new adsorbents with high adsorption capacities is very important for removal of the OPE pollutants in the environment.[13]
Banning of Diazinon
During the Clinton Era, President Clinton signed a tougher pesticide law in 1996. Diazinon was banned from use as an agricultural insecticide. At the time 80% of United States could find Diazinon in their residential products. In fact it still may be in use in certain households, as it is still used and considered legal in 14 states including California. Yet with the ban starting in 2004, having had 20 years pass, states which have accepted that ban have had hardware stores and other suppliers report that they have "ran out" of products with diazinon. These states which continue to use Diazinon is their products consider their risks low, yet its greatest affect is through inhalation and skin contact. Certain environmental groups continue to protest these states which still continue its use.[14]
Toxicity and effects on animals
[edit]Diazinon is considered to be of relatively hightoxicityforvertebrates.The common method of administering diazinon is absorption although inhalation is possible as well. The observed toxification symptoms conform to otheracetylcholinesterase inhibitors.Symptoms are as follows:
- Colic
- Diarrheaand/orvomiting
- Vertigo
- Headaches
- Miosis
- Bradycardia
- Suddendrop in blood pressure
- Convulsion
- Apnea
| Lethal Dose | Observations |
|---|---|
| LD50 |
|
On the other hand, in regard tochronic toxicity,the WHO/FAO joint committee on pesticide residues gives the admissible daily intake (ADI) to be 0.005 mg/kg of body weight, while the Australian Pesticides and Veterinary Medicine authority gives the no-observed-adverse-effect-level (NOAEL) to be 0.02 mg/kg of body weight for adults.
Symptoms in humans
[edit]Intoxication of diazinon produces the following signs and symptoms:
- Eyes, ears, nose, and throat
- Small pupils (unreactive to light)
- Tearing, increased
- Cardiovascular
- Low or high blood pressure
- Slow or rapid heart rate
- Respiratory
- Breathing difficulty
- Chest tightness
- Nervous system
- Anxiety
- Convulsions
- Coma
- Dizziness
- Excitability
- Headache
- Weakness
- Tremor
- Twitching
- Skin
- Irritation
- Redness
- Sweating
- Gastrointestinal
- Abdominal cramps
- Diarrhea
- Loss of appetite
- Nausea
- Vomiting[citation needed]
Typically treatments will vary depending on exposure and method of administration of the toxin. Critical biomarkers such as urine samples, blood content and heart rates are measured while detoxifying the patient. Common treatments for patients with diazinon poisoning include:
- Assisted Breathing
- Intravenous fluids (IV)
- Irrigation (washing of the skin and eyes)
- Medicinal Treatments; including the antidotesatropineandoxime.[15]
- Gastric Lavage[citation needed]
Patients that continue to improve over the first 4 to 6 hours (after medical treatment) usually recover unscathed. Prolonged treatment often is needed to reverse the poisoning, including intensive care hospitalization and long-term therapy. Some toxicity may persist for weeks or months, or even longer.[citation needed]
Efficacy and side effects
[edit]Diazinon is a contact insecticide which kills insects by altering normalneurotransmissionwithin the nervous system of the insect. As mentioned above, diazinon inhibits the enzyme acetylcholinesterase (AChE), which hydrolyzes the neurotransmitter acetylcholine (ACh) incholinergicsynapsesandneuromuscular junctions.This results in abnormal accumulation of ACh within the nervous system. Diazinon, although athiophosphoric ester,shares a common mechanism of toxicity with other organophosphate insecticides such aschlorpyrifos,malathionandparathion,and is not very effective against the organophosphate-resistant insect populations.[citation needed]
Symptoms of acute diazinon exposure develop in minutes to hours following exposure, depending on the exposure pathway. The initial symptoms of humans are nausea, dizziness, salivation, headache, sweating,lacrimation,andrhinorrhea.The symptoms can progress to vomiting, abdominal cramps, diarrhea, muscle twitching, weakness, tremor, a lack of coordination andmiosis.Furthermore, some studies have even reported some psychiatric side effects including memory loss, confusion, and depression.[citation needed]
Because diazinon is fat soluble, there is potential for delayed toxicity if significant amounts of diazinon are stored in fatty tissues. Intermediate syndrome generally occurs within 24–96 hours after exposure. Intermediate syndrome in humans is characterized by difficulty breathing and muscular weakness, often in the face, neck and proximal limb muscles. Cranial nerve palsies and depressed tendon reflexes have also been reported.[citation needed]
Studies have suggested that exposure to some organophosphate pesticides can result in long-term neurological problems including organophosphate-induced delayed neuropathy (weakness or paralysis as well asparesthesiain the extremities); however, reports of these symptoms following diazinon exposures are rare. Human who have been poisoned show increased levels of serumamylaseand glucose as well as elevated urinarydiastaselevels accompanied by symptoms considered to be indicative of acutepancreatitis.[citation needed]
A study found that 10% of 21 typically developingchildrenshow 2-isopropyl-6-methyl-4-pyrimidinol (IMPy, a metabolite of diazinon) inmolars.Molars from the two oldest subjects contained the largest concentrations of IMPy. And this concentration in molars may be a biomarker of perinatal exposure and during molar formation.[16]
References
[edit]- ^Budavari, S., ed. (1996).The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals.Whitehouse Station, NJ: Merck. p. 508.
- ^abcdeNIOSH Pocket Guide to Chemical Hazards."#0181".National Institute for Occupational Safety and Health(NIOSH).
- ^Sharom, M.S.; Miles, J.R.W.; Harris, C.R.; McEwen, F.L. (1980). "Behaviour of 12 insecticides in soil and aqueous suspensions of soil and sediment".Water Research.14(8): 1095–100.Bibcode:1980WatRe..14.1095S.doi:10.1016/0043-1354(80)90158-X.
- ^Hansch, Corwin; Leo, Albert; Hoekman, David (1995).Exploring QSAR: Volume 2: Hydrophobic, Electronic, and Steric Constants.Washington, DC: American Chemical Society. p. 106.ISBN978-0-8412-2991-4.
- ^abCone, Marla (1 Jan 2005)."EPA Takes Pest Killer Diazinon Off the Shelves".Los Angeles Times.Retrieved2 Jul2020.
- ^Geller, Robert J.; Lopez, Gaylord P.; Cutler, Stephen; Lin, Diana; Bachman, George F.; Gorman, Susan E. (2003). "Atropine availability as an antidote for nerve agent casualties: Validated rapid reformulation of high-concentration atropine from bulk powder".Annals of Emergency Medicine.41(4): 453–6.doi:10.1067/mem.2003.103.PMID12658242.
- ^"Diazinon General Fact Sheet".npic.orst.edu.Retrieved2024-03-14.
- ^Dahlgren, J. G.; Takhar, H. S.; Ruffalo, C. A.; Zwass, M. (2004)."Health effects of diazinon on a family".Journal of Toxicology. Clinical Toxicology.42(5): 579–591.doi:10.1081/clt-200026979.ISSN0731-3810.PMID15462149.
- ^"Diazinon | Public Health Statement | ATSDR".wwwn.cdc.gov.Retrieved2024-03-14.
- ^"Diazinon | Public Health Statement | ATSDR".wwwn.cdc.gov.Retrieved2024-03-14.
- ^"Diazinon Technical Fact Sheet".National Pesticide Information Center.NPIC.Retrieved31 May2019.
- ^Kretschmann, Andreas; et al. (2011). "Mechanistic Toxicodynamic Model for Receptor-Mediated Toxicity of Diazoxon, the Active Metabolite of Diazinon, in Daphnia magna".Environmental Science & Technology.45(11): 4980–4987.Bibcode:2011EnST...45.4980K.doi:10.1021/es1042386.PMID21539304.S2CID31463849.
- ^Amani, M. A; Latifi, A. M; Tahvildari, K; Karimian, R (2017). "Removal of diazinon pesticide from aqueous solutions using MCM-41 type materials: Isotherms, kinetics and thermodynamics".International Journal of Environmental Science and Technology.15(6): 1301–1312.doi:10.1007/s13762-017-1469-x.S2CID104194226.
- ^Cone, Marla (2005-01-01)."EPA Takes Pest Killer Diazinon Off the Shelves".Los Angeles Times.Retrieved2024-03-14.
- ^Office of Chemical Safety Department of Health and Ageing Canberra."AUSTRALIAN PESTICIDES AND VETERINARY MEDICINES AUTHORITY"(PDF).apvma.gov.au.Archived fromthe original(PDF)on 2013-04-19.Retrieved2024-05-14.
- ^Camann, David E.; Schultz, Stephen T.; Yau, Alice Y.; Heilbrun, Lynne P.; Zuniga, Michelle M.; Palmer, Raymond F.; Miller, Claudia S. (March 2013)."Acetaminophen, pesticide, and diethylhexyl phthalate metabolites, anandamide, and fatty acids in deciduous molars: potential biomarkers of perinatal exposure".Journal of Exposure Science and Environmental Epidemiology.23(2): 190–196.doi:10.1038/jes.2012.71.ISSN1559-0631.PMID22805989.
External links
[edit]- Diazinon general information-National Pesticide Information Center
- Diazinon Technical Fact Sheet - National Pesticide Information Center
- Diazinon Pesticide Information Profile - Extension Toxicology Network
- EPA Documents: Diazinon
- CDC - NIOSH Pocket Guide to Chemical Hazards - Diazinon
- Chemical Fact Sheet
- Diazinon,Public Health Statement
