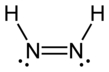Diimide
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Diazene
| |||
| Other names
Diimide
Diimine Dihydridodinitrogen Azodihydrogen | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Diazene | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| H2N2 | |||
| Molar mass | 30.030g·mol−1 | ||
| Appearance | Yellow gas | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Related compounds | |||
Otheranions
|
diphosphene dinitrogen difluoride | ||
Othercations
|
azo compounds | ||
Related Binaryazanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
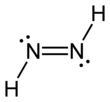
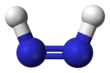
Diimide,also calleddiazeneordiimine,is a compound having the formula HN=NH. It exists as twogeometric isomers,E(trans) andZ(cis). The term diazene is more common for organic derivatives of diimide. Thus,azobenzeneis an example of an organic diazene.
Synthesis
[edit]A traditional route to diimide involves oxidation ofhydrazinewith hydrogen peroxide or air.[1]
- N2H4+ H2O2→ N2H2+ 2H2O
Alternatively the hydrolysis ofdiethyl azodicarboxylateorazodicarbonamideaffords diimide:[2]
- Et−O2C−N=N−CO2−Et → HN=NH + 2 CO2+ 2 HOEt
Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide.[3]
Because of its instability, diimide is generated and usedin-situ.A mixture of both thecis(Z-) andtrans(E-) isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. Thetransisomer is more stable, but thecisisomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them shifts towards thecisisomer due toLe Chatelier's principle.Some procedures call for the addition of carboxylic acids, which catalyse the cis–trans isomerization.[4]Diimide decomposes readily. Even at low temperatures, the more stabletransisomer rapidly undergoes various disproportionation reactions, primarily forminghydrazineandnitrogen gas:[5]
- 2 HN=NH → H2N−NH2+ N2
Because of this competing decomposition reaction, reductions with diimide typically require a large excess of the precursor reagent.
Applications to organic synthesis
[edit]Diimide is occasionally useful as a reagent inorganic synthesis.[4]It hydrogenates alkenes and alkynes with selective delivery of hydrogen from one face of the substrate resulting in the same stereoselectivity as metal-catalysedsynaddition ofH2.The only coproduct released is nitrogen gas. Although the method is cumbersome, the use of diimide avoids the need for high pressures or hydrogen gas and metal catalysts, which can be expensive.[6]The hydrogenation mechanism involves a six-memberedC2H2N2transition state:
Selectivity
[edit]Diimide is advantageous because it selectively reduces alkenes and alkynes and is unreactive toward manyfunctional groupsthat would interfere with normalcatalytic hydrogenation.Thus,peroxides,alkyl halides,andthiolsare tolerated by diimide, but these same groups would typically be degraded by metal catalysts. The reagent preferentially reduces alkynes and unhindered or strained alkenes[1]to the corresponding alkenes and alkanes.[4]
Related
[edit]Thedicationicform,H−N+≡N+−H(diazynediium, diprotonated dinitrogen), is calculated to have the strongest known chemical bond. This ion can be thought of as a doubly protonated nitrogen molecule. Therelative bond strength order(RBSO) is 3.38.[7]F−N+≡N+−H(fluorodiazynediium ion) andF−N+≡N+−F(difluorodiazynediium ion) have slightly lower strength bonds.[7]
In the presence of strong bases, diimide deprotonates to form thepernitrideanion,N−=N−.
References
[edit]- ^abOhno, M.; Okamoto, M. (1973)."cis-Cyclododecene".Organic Syntheses;Collected Volumes,vol. 5, p. 281.
- ^Wiberg, E.; Holleman, A. F. (2001)."1.2.7: Diimine, N2H2".Inorganic Chemistry.Elsevier.p. 628.ISBN9780123526519.
- ^Chamberlin, A. Richard; Sheppeck, James E.; Somoza, Alvaro (2008). "2,4,6-Triisopropylbenzenesulfonylhydrazide".Encyclopedia of Reagents for Organic Synthesis.doi:10.1002/047084289X.rt259.pub2.ISBN978-0471936237.
- ^abcPasto, D. J. (2001). "Diimide".Encyclopedia of Reagents for Organic Synthesis.John Wiley & Sons.doi:10.1002/047084289X.rd235.ISBN0471936235.
- ^Wiberg, Nils; Holleman, A. F.; Wiberg, Egon, eds. (2001). "1.2.7 Diimine N2H2[1.13.17] ".Inorganic Chemistry.Academic Press. pp. 628–632.ISBN978-0123526519.
- ^Miller, C. E. (1965). "Hydrogenation with Diimide".Journal of Chemical Education.42(5): 254–259.Bibcode:1965JChEd..42..254M.doi:10.1021/ed042p254.
- ^abKalescky, Robert; Kraka, Elfi; Cremer, Dieter (12 September 2013). "Identification of the Strongest Bonds in Chemistry".The Journal of Physical Chemistry A.117(36): 8981–8995.Bibcode:2013JPCA..117.8981K.doi:10.1021/jp406200w.PMID23927609.S2CID11884042.