Eimeria
| Eimeria | |
|---|---|

| |
| Oocysts of various species | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Conoidasida |
| Order: | Eucoccidiorida |
| Family: | Eimeriidae |
| Genus: | Eimeria Schneider,1875 |
| Type species | |
| Eimeria falciformis[1] (Eimer, 1870) Schneider, 1875
| |
| Species | |
|
See text | |
Eimeriais agenusofapicomplexanparasites that includes various species capable of causing the diseasecoccidiosisin animals such ascattle,poultryand smallerruminantsincludingsheepandgoats.[2]Eimeriaspecies are considered to bemonoxenousbecause the life cycle is completed within a single host, and stenoxenous because they tend to be host specific, although a number of exceptions have been identified. Species of this genus infect a wide variety of hosts. Thirty-one species are known to occur inbats(Chiroptera), two in turtles, and 130 named species infect fish. Two species (E. phocaeandE. weddelli) infect seals. Five species infect llamas and alpacas:E. alpacae,E. ivitaensis,E. lamae,E. macusaniensis,andE. punonensis.A number of species infect rodents, includingE. couesii,E. kinsellai,E. palustris,E. ojastiiandE. oryzomysi.Others infect poultry (E. necatrixandE. tenella), rabbits (E. stiedai) and cattle (E. bovis,E. ellipsoidalis,andE. zuernii).[3]For full species list, see below.
The most prevalent species ofEimeriathat cause coccidiosis in cattle areE. bovis,E. zuernii,andE. auburnensis.In a young, susceptible calf it is estimated that as few as 50,000 infectiveoocystscan cause severe disease.[4]Eimeriainfections are particularly damaging to the poultry industry and costs the United States more than $1.5 billion in annual losses.[5]The most economically important species among poultry areE. tenella,E. acervulina,andE. maxima.[6]The oocysts of what was later calledEimeria stiedaiwere first seen by the pioneering Dutch microscopistAntonie van Leeuwenhoek(1632–1723) in thebileof arabbitin 1674. The genus is named after the GermanzoologistTheodor Eimer(1843–1898).
Life cycle
[edit]TheEimerialife cycle has anexogenousphase, during which the oocysts are excreted into the environment, and anendogenousphase, where parasite development occurs in the host intestine. During the endogenous phase, several rounds ofschizogony(asexual reproduction) take place, after which thesexual differentiationofgametesandfertilisationoccurs. Parasite transmission occurs via thefecal-oral route.Infections are common in farming environments where many animals are confined in a small space.[7]

Oocysts
[edit]There are two forms ofoocyst:sporulated or late oocyst, and unsporulated or early oocyst. An infected host releases oocysts into the environment in their unsporulated form. These contain a multi-layered cell wall making them highly resistant to environmental pressures.[8]Once released, the unsporulated oocysts undergomeiosisupon contact with oxygen and moisture.[9]This process is known assporulationand the oocysts take approximately 2 to 7 days to become infectious.[10]The sporulated oocyst is said to betetrasporicmeaning it contains four sporocysts, while each sporocyst isdizoic,i.e. it contains two sporozoites.[3]
Once ingested, the oocysts undergo a process called excystation, whereby thousands of sporozoites are released into lumen of the intestine. In the case ofE. tenella,this process is thought to occur due to the combination of enzymatic degradation and mechanical abrasion of the oocyst wall in the chicken'sgizzard.[11]
Sporozoites
[edit]The motilesporozoitesinvade theenterocytesof small intestine, and migrate to their respective sites of development. Invasion is mediated through specialised membrane-bound structures on the surface of the parasite that release secretions. This results in the recognition of, and attachment tohost cellreceptors.This process is known asgliding motility,which is conserved across all species ofApicomplexa.Membrane glyconjugates have been proposed as potential host cell receptors forEimeriaspecies.[12]After invasion, the sporozoites develop intotrophozoites,then intoschizonts,where they undergo several rounds ofasexual reproduction.This results in manynucleideveloping within the schizont. Each nucleus develops into amerozoite.[3]
Invasion requires the formation of amoving junctionbetween parasite and hostcell membranes.InE. tenella,this involves parasitemicronemesandrhoptryproteins including RON2, RON5 and AMA-2.[11]It is unlikely that the host cell is completely passive in the invasion process, although evidence of host physical forces that assist in mediating parasite entry remains controversial.
Merozoites
[edit]Whenschizontsrupture, merozoites are released, which either go on to re-infect moreenterocytesor develop into either male or femalegametesvia the process ofgametogenesis.These gametes fuse to form an oocyst, which is then released in its non-infectious, unsporulated form through the faeces of the host.
Merozoite invasion also requires the formation of a moving junction, however theproteinsinvolved in this process differs from those on sporozoites.Rhoptryproteins AMA-1 and RON4 are found exclusively on merozoites. There is also a greater diversity ofvariant surface antigensfound on the surface of merozoites. It is hypothesised that this may be due to the fact that merozoites are short-lived and a greaterantigenrepertoire would permit faster binding and invasion.[11]
Taxonomy
[edit]
TheEimerialie within the familyEimeriidae.Eimeriaaccounts for close to 75% of the species within this family, and it is the most specious of the genera of theApicomplexawith 1,700 described species.[13]
Attempts to subdivide this large taxonomic unit into separate genera have been made. The classification of eimeriid coccidian was largely based on morphological and life cycle details.[14]More recently, classification has been done usingrDNAandmitochondrial genes,which indicateEimeriamay beparaphyletictoIsosporaandCyclospora[15]
Eimeria:These species are tetrasporocystic with dizoic, nonbivalved sporocysts with or withoutStieda bodies.This new genus retains the majority of the species.[13]
Goussia(Labbe 1896): These species are tetrasporocystic, dizoic, lack Stieda bodies, and have sporocyst walls consisting of two valves joined by a longitudinal suture. This genus contains about 20 species.[16]
Crystallospora(Thelohan 1893): The species in this genus is tetrasporocystic and dizoic, and have dodecahedral sporocysts composed of two hexagonal, pyrimidal valves joined at their bases by a suture. This genus contains a single species,Crystallospora cristalloides
Epieimeria:The species in this genus are tetrasporocystic, dizoic, possess Stieda bodies, and undergo merogony and gametogony on the lumenal surface of the intestinal tract. Three species are in this genus.[17]
Species identification
[edit]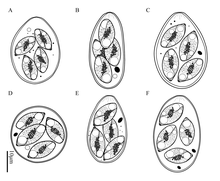
Methods for species identification are varied and among others, includeisozymeanalysis,[18]the use ofrRNAandrDNAprobes,[19]DNAassaysandrecombinant DNAtechniques.[20][21][22]PCRhas proven most useful foroutbreaksurveillance.[23]Prior to these methods, species identification was based onphenotypiccharacteristics such as the site of parasite development, the oocyst structure, the host species, crossimmunityand the presence of lesions. Out of these, comparing oocyst structures was the most commonly used method.[24][3]
Genomics
[edit]Awhole genome sequencingproject is in progress with chosen species,Eimeria tenella.The genome is about 60megabasesin size and has aGC-contentaround 53%. The 14chromosomesrange in size from 1 to > 6 megabases. Since 2013, the sequencing and annotation of a further six avianEimeriaspecies genomes is in progress.[25]
Pathology and symptoms
[edit]Coccidiosis typically results indiarrhoea,weight loss anddehydration.A combination of these factors may result in poor growth and death of the animal, particularly amongst young.[10]Other clinical signs includelethargy,depression,and reduced normal grooming behaviour.[26]Diarrhoea may be bloody due tointestinal epitheliumdying off when a large number of oocysts and merozoites burst out of the cells.
The severity of the disease is directly dependent on the number of infectiveEimeriaoocysts that are ingested.[27]Thepathogenesisof infection varies from mild to severe, and is largely dependent on the magnitude of infection.[28]In light infections, the damage to the gut might only be minimal and be rapidly repaired as cells are rapidly replaced by the body. However, in heavy infections, it may only take two weeks for many intestinal epithelial cells to be infected with eitherEimeriameronts or gametocytes. These cause the epithelial cells to burst, which causes significant damage to the intestine epithelial layer, resulting in the release of blood, fluid, andelectrolytesinto the intestine.[29]
Prevention and treatment
[edit]Goodanimal husbandrypractices andprophylacticapplication of anticoccidial drugs that target different stages of the parasite lifecycle, such assulfonamides,ionophoresandtoltrazuril,are the preferred methods of disease prevention, particularly in the poultry industry.[7][2]
The following drugs can be used for treatment of coccidiosis in cattle:amprolium,sulfaquinoxaline,andsulfamethazine.However, it is often more effective to prevent this disease in cattle, which can be aided by the productslasalocid,decoquinate,andmonensin.[29]
There is a growing problem ofdrug resistance,as well as possible drug residues in the meat once the animal is butchered. As a result, other avenues of control are being explored, particularly vaccine development, although severallive attenuated vaccineshave been in use since the 1950s.[30][31]So far, the best practice is to vaccinate the chicks once they hatch from the egg so they are immune for life.
Infection withEimeriaresults in life-longimmunityto that particular parasite species, but does not give cross protection against other species. For these reasons, vaccines for control seem promising, of which live attenuated vaccines are most effective. However, the search for highlyimmunogenicantigensand overcomingantigenic variationof the parasites remains a challenge. Immunity to the parasite varies depending on parasite and host species, as well as the site of invasion.CD4+ T cellsandinterferon gamma(γ) are crucial components ofnatural immunityto infection.[32]Humoral immunityis thought to play little role in protection, and is most likely mediated through secretoryIgAantibodies.[12]
Host-parasite relations
[edit]Fish
[edit]- Eimeria aurati-goldfish(Carassius auratus)
- Eimeria baueri-crucian carp(Carassius carassius)
- Eimeria lepidosirenis-South American lungfish(Lepidosiren paradoxa)
- Eimeria leucisci-common barbel(Barbus barbus bocagei)
- Eimeria rutili-European chub(Leuciscus cephalus cabeda),Iberian nase(Chondrostoma polylepis polylepis)
- Eimeria vanasi- blue tilapia (Oreochromis aureus)
Reptiles
[edit]- Eimeria amphisbaeniarum- Mann'sworm lizard(Amphisbaena manni)
- Eimeria witcheri- Mann's worm lizard (A. manni)
- Eimeria yemenensae- rock agama (Agama yemenensis)
Birds
[edit]- Eimeria acervulina-chicken(Gallus gallus domesticus)
- Eimeria adenoeides-turkey(Meleagris gallopavo)
- Eimeria brunetti- chicken (G. g. domesticus)
- Eimeria colchici-common pheasant(Phasianus colchicus)
- Eimeria curvata-ruddy ground dove(Columbina talpacoti),scaled dove(Scardafella squammata)
- Eimeria dispersa- turkey (M. gallopavo), bobwhite quail (Colinus virginianus)
- Eimeria duodenalis- pheasant (Phasianus colchicus)
- Eimeria fraterculae- Atlantic puffin (Fratercula arctica)
- Eimeria gallopavonis- turkey (M. gallopavo)
- Eimeria innocua- turkey (M. gallopavo)
- Eimeria praecox- chicken (G. g. domesticus)
- Eimeria maxima- chicken (G. g. domesticus)
- Eimeria meleagridis- turkey (M. gallopavo)
- Eimeria meleagrimitis- turkey (M. gallopavo)
- Eimeria mitis- chicken (G. g. domesticus)
- Eimeria muta-rock ptarmigan(Lagopus muta islandorum)[33]
- Eimeria necatrix- chicken (G. g. domesticus)
- Eimeria phasiani- pheasant (P. colchicus)
- Eimeria procera- grey partridges (Perdix perdix)
- Eimeria purpureicephali- red-capped parrot (Purpureicephalus spurius)[34]
- Eimeria rjupa-rock ptarmigan(L. m. islandorum)
- Eimeria tenella- chicken (G. g. domesticus)
Mammals
[edit]- Eimeria ahsata- goat (Capra hircus), sheep (Ovis aries)
- Eimeria alabamensis- cattle (Bos taurus)
- Eimeria alijevi- goat (C. hircus)
- Eimeria aspheronica- goat (C. hircus)
- Eimeria arloingi- goat (C. hircus)
- Eimeria arundeli- common wombat (Vombatus ursinus)
- Eimeria bakuensis- sheep (O. aries)
- Eimeria bovis- cattle (B. taurus)
- Eimeria cameli- camels (Camelus bactrianus,Camelus dromedarius)
- Eimeria caprina- goat (C. hircus)
- Eimeria caprovina- goat (C. hircus)
- Eimeria christenseni- goat (C. hircus)
- Eimeria clethrionomyis- red-backed vole (Clethrionomys gapperi)
- Eimeria coecicola- rabbit (Oryctolagus cuniculus)
- Eimeria contorta- mouse (Mus musculus)
- Eimeria couesii- rice rat (Oryzomys couesi)
- Eimeria crandallis- sheep (O. aries)
- Eimeria dammahensis- scimitar-homed oryx (Oryx dammah)
- Eimeria dowleri- eastern red bat (Lasiurus borealis)
- Eimeria exigua- rabbit (O. cuniculus)
- Eimeria falciformis- mouse (M. musculus)
- Eimeria farasanii- mountain gazelle (Gazella gazelle farasani)
- Eimeria ferrisi- mouse (M. musculus)
- Eimeria flavescens- rabbit (O. cuniculus)
- Eimeria gallatii- red-backed vole (C. gapperi)
- Eimeria granulosa- goat (C. hircus)
- Eimeria hirci- goat (C. hircus)
- Eimeria intestinalis- rabbit (O. cuniculus)
- Eimeria irresidua- rabbit (O. cuniculus)
- Eimeria intricata- goat (C. hircus)
- Eimeria jolchijevi- goat (C. hircus)
- Eimeria krijgsmanni- mouse (M. musculus)
- Eimeria larimerensis- Uinta ground squirrel (Spermophilus armatus)
- Eimeria macusaniensis- llamas (Lama glama), guanacos (Lama guanicoe), alpacas (Vicugna pacos), vicunas (Vicugna vicugna)
- Eimeria magna- rabbit (O. cuniculus)
- Eimeria marconii- red-backed vole (Clethrionomys gapperi)
- Eimeria media- rabbit (O. cuniculus)
- Eimeria melanuri- garden dormouse (Eliomys quercinus)
- Eimeria myoxi- garden dormouse (E. quercinus)
- Eimeria nagpurensis- rabbit (O. cuniculus)
- Eimeria nieschulzi- brown rat (R. norvegicus)
- Eimeria ninakohlyakimovae- goat (C. hircus)
- Eimeria ovinoidalis- sheep (O. aries)
- Eimeria pallida- goat (C. hircus)
- Eimeria palustris- marsh rice rat (Oryzomys palustris)
- Eimeria papillata- mouse (M. musculus)
- Eimeria perforans- rabbit (O. cuniculus)
- Eimeria phocae-Sable Islandharbour seals (Phoca vitulina)
- Eimeria pileata- red-backed vole (Clethrionomys gapperi)
- Eimeria pipistrellus- Kuhl's pipistrelle (Pipistrellus kuhlii)
- Eimeria piriformis- rabbit (O. cuniculus)
- Eimeria prionotemni- Bennett's wallaby (Macropus rufogriseus)
- Eimeria procyonis- raccoon (Procyon lotor)
- Eimeria punctata- goat (C. hircus)
- Eimeria roobroucki- rabbit (O. cuniculus)
- Eimeria saudiensis- Arabian oryx (Oryx leucoryx)
- Eimeria sealanderi- eastern red bat (Lasiurus borealis)
- Eimeria separata- mouse (M. musculus), rat (Rattus rattus)
- Eimeria stiedai- rabbit (O. cuniculus)
- Eimeria ursini- southern hairy nosed wombat (Lasiorhinus latifrons)
- Eimeria vermiformis- mice (M. musculus)
- Eimeria weybridgensis- sheep (O. aries)
- Eimeria wobati- southern hairy-nosed wombat (L. latifrons)
- Eimeria zuernii- cattle (B. taurus)
List of species
[edit]- Eimeria abramovi
- Eimeria acervulina
- Eimeria adenoides
- Eimeria ahsata
- Eimeria airculensis
- Eimeria alabamensis
- Eimeria albigulae
- Eimeria alijevi
- Eimeria alpacae
- Eimeria amphisbaeniarum
- Eimeria anatis
- Eimeria anguillae
- Eimeria ankarensis
- Eimeria anseris
- Eimeria arizonensis
- Eimeria arabukosokokensis
- Eimeria arnyi
- Eimeria arundeli
- Eimeria anseris
- Eimeria arkhari
- Eimeria arloingi
- Eimeria aspheronica
- Eimeria auburnensis
- Eimeria augusta
- Eimeria aurati
- Eimeria aythyae
- Eimeria azerbaidschanica
- Eimeria bactriani
- Eimeria bakuensis
- Eimeria bareillyi
- Eimeria baueri
- Eimeria battakhi
- Eimeria beckeri
- Eimeria beecheyi
- Eimeria berkinbaevi
- Eimeria brinkmanni
- Eimeria bombaynsis
- Eimeria bonasae
- Eimeria boschadis
- Eimeria bovis
- Eimeria brantae
- Eimeria brasiliensis
- Eimeria brevoortiana
- Eimeria brinkmanni
- Eimeria brunetti
- Eimeria bucephalae
- Eimeria bufomarini
- Eimeria bukidnonensis
- Eimeria burdai
- Eimeria callospermophili
- Eimeria californicenis
- Eimeria cameli
- Eimeria canadensis
- Eimeria canis
- Eimeria caprina
- Eimeria caprovina
- Eimeria carinii
- Eimeria carpelli
- Eimeria catostomi
- Eimeria catronensis
- Eimeria caviae
- Eimeria cerdonis
- Eimeria citelli
- Eimeria chelydrae
- Eimeria christenseni
- Eimeria clarkei
- Eimeria clethrionomyis
- Eimeria coecicola
- Eimeria colchici
- Eimeria columbae
- Eimeria columbarum
- Eimeria contorta
- Eimeria coturnicus
- Eimeria couesii
- Eimeria crandallis
- Eimeria crassa
- Eimeria curvata
- Eimeria cylindrica
- Eimeria cynomysis
- Eimeria cyprini
- Eimeria dammahensis
- Eimeria danailovi
- Eimeria danielle
- Eimeria debliecki
- Eimeria deserticola
- Eimeria dispersa
- Eimeria dolichotis
- Eimeria dromedarii
- Eimeria duszynskii
- Eimeria ellipsoidalis
- Eimeria elongata
- Eimeria etheostomae
- Eimeria eutamiae
- Eimeria exigua
- Eimeria falciformis
- Eimeria fanthami
- Eimeria farasanii
- Eimeria farra
- Eimeria faurei
- Eimeria fernandoae
- Eimeria ferrisi
- Eimeria filamentifera
- Eimeria franklinii
- Eimeria fraterculae
- Eimeria freemani
- Eimeria fulva
- Eimeria funduli
- Eimeria gallatii
- Eimeria gallopavonis
- Eimeria gasterostei
- Eimeria gilruthi
- Eimeria glenorensis
- Eimeria gokaki
- Eimeria gonzalei
- Eimeria gorakhpuri
- Eimeria granulosa
- Eimeria grenieri
- Eimeria guevarai
- Eimeria hagani
- Eimeria haneki
- Eimeria hasei
- Eimeria hawkinsi
- Eimeria hermani
- Eimeria hindlei
- Eimeria hirci
- Eimeria hoffmani
- Eimeria hoffmeisteri
- Eimeria hybognathi
- Eimeria ictaluri
- Eimeria illinoisensis
- Eimeria innocua
- Eimeria intestinalis
- Eimeria intricata
- Eimeria iroquoina
- Eimeria irresidua
- Eimeria ivitaensis
- Eimeria judoviciani
- Eimeria kinsellai
- Eimeria koganae
- Eimeria kotlani
- Eimeria krijgsmanni
- Eimeria krylovi
- Eimeria kunmingensis
- Eimeria lagopodi
- Eimeria lamae
- Eimeria langebarteli
- Eimeria larimerensis
- Eimeria lateralis
- Eimeria laureleus
- Eimeria lepidosirenis
- Eimeria leucisci
- Eimeria ludoviciani
- Eimeria macusaniensis
- Eimeria magnalabia
- Eimeria marconii
- Eimeria maxima
- Eimeria melanuri
- Eimeria meleagridis
- Eimeria menzbieri
- Eimeria micropteri
- Eimeria minasensis
- Eimeria mitis
- Eimeria monacis
- Eimeria morainensis
- Eimeria moronei
- Eimeria mulardi
- Eimeria muta
- Eimeria myoxi
- Eimeria myoxocephali
- Eimeria natricis
- Eimeria necatrix
- Eimeria neitzi
- Eimeria nieschulzi
- Eimeria nigricani
- Eimeria nocens
- Eimeria nyroca
- Eimeria ojastii
- Eimeria ojibwana
- Eimeria onychomysis
- Eimeria oryzomysi
- Eimeria oryxae
- Eimeria os
- Eimeria osmeri
- Eimeria ovata
- Eimeria ovinoidalis
- Eimeria palustris
- Eimeria papillata
- Eimeria parvula
- Eimeria pigra
- Eimeria pilarensis
- Eimeria pileata
- Eimeria pipistrellus
- Eimeria phocae
- Eimeria praecox
- Eimeria prionotemni
- Eimeria pseudospermophili
- Eimeria pulchella
- Eimeria pungitii
- Eimeria punonensis
- Eimeria ranae
- Eimeria reedi
- Eimeria reichenowi
- Eimeria ribarrensis
- Eimeria rjupa
- Eimeria rutili
- Eimeria salvelini
- Eimeria saitamae
- Eimeria saudiensis
- Eimeria separata
- Eimeria schachdagica
- Eimeria sevilletensis
- Eimeria sinensis
- Eimeria sipedon
- Eimeria somateriae
- Eimeria spermophili
- Eimeria squali
- Eimeria stiedai
- Eimeria stigmosa
- Eimeria striata
- Eimeria subepithelialis
- Eimeria surki
- Eimeria tamiasciuri
- Eimeria tedlai
- Eimeria tenella
- Eimeria truncata
- Eimeria truttae
- Eimeria uekii
- Eimeria uniungulati
- Eimeria ursini
- Eimeria vilasi
- Eimeria weddelli
- Eimeria weybridgensis
- Eimeria witcheri
- Eimeria vanasi
- Eimeria vermiformis
- Eimeria volgensis
- Eimeria wobati
- Eimeria wyomingensis
- Eimeria yemenensae
- Eimeria yukonensis
- Eimeria zuernii
References
[edit]- ^Donald W. Duszynski, Steve J. Upton & Lee Couch."Taxonomic Summary of Genera within the Eimeriidae".University of New Mexico.Archived fromthe originalon 4 October 2013.RetrievedNovember 8,2010.
- ^abChartier, Paraud (2012). "Coccidiosis due to Eimeria in sheep and goats, a review".Small Ruminant Research.103(1): 84–92.doi:10.1016/j.smallrumres.2011.10.022.
- ^abcdFayner R (1980). "Epidemiology of protozoan infections: the coccidia".Veterinary Parasitology.6(1–3): 75–103.doi:10.1016/0304-4017(80)90039-4.
- ^Daugschies A, Najdrowski M (2005). "Eimeriosis in Cattle: Current Understanding".Veterinary Medicine.52(10): 417–427.doi:10.1111/j.1439-0450.2005.00894.x.PMID16364016.
- ^Yun CH, Lillehoj HS, Lillehoj EP (2002). "Intestinal immune responses to coccidiosis".Developmental & Comparative Immunology.24(2–3): 303–324.doi:10.1016/S0145-305X(99)00080-4.PMID10717295.
- ^Shirley MW, Ivens A, Gruber A, et al. (2004). "The Eimeria genome projects: a sequence of events".Trends in Parasitology.20(5): 199–201.doi:10.1016/j.pt.2004.02.005.PMID15105014.
- ^abMcDonald V, Shirley MW (2009). "Past and future: vaccination againstEimeria".Parasitology.136(12): 1477–1489.doi:10.1017/S0031182009006349.PMID19523251.S2CID43277313.
- ^Belli SI, Smith NC, Ferguson DJ (2006). "The coccidian oocyst: a tough nut to crack!".Trends in Parasitology.22(9): 416–423.doi:10.1016/j.pt.2004.02.005.PMID15105014.
- ^Ryan R, Shirley M, Tomley F (2000). "Mapping and expression of micronemes genes inEimeria tenella".International Journal for Parasitology.30(14): 1493–1499.doi:10.1016/s0020-7519(00)00116-8.PMID11428341.
- ^abForeyt WJ (1990). "Coccidiosis and Cryptosporidiosis in Sheep and Goats".Veterinary Clinics of North America: Food Animal Practice.6(3): 655–670.doi:10.1016/S0749-0720(15)30838-0.PMID2245367.
- ^abcLal K; et al. (2009)."Proteomic comparison of fourEimeria tenellalife-cycle stages: Unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite ".Proteomics.9(19): 4566–4576.doi:10.1002/pmic.200900305.PMC2947549.PMID19795439.
- ^abAugustine PC (2000)."Cellular invasion by avianEimeriaspecies ".Poultry and Avian Biology Reviews.11(1): 113–122.doi:10.1016/S0020-7519(00)00150-8.PMID11286188.
- ^abOgedengbe JD, Ogedengbe ME, Hafeez MA, Barta JR (2015). "Molecular phylogenetics of eimeriid coccidia (Eimeriidae, Eimeriorina, Apicomplexa, Alveolata): A preliminary multi-gene and multi-genome approach".International Journal for Parasitology.41(8): 843–850.doi:10.1016/j.ijpara.2011.03.007.PMID21515277.
- ^Levine ND (1988). "Progress in Taxonomy of the Apicomplexan Protozoa".Eukaryotic Microbiology.35(4): 518–520.doi:10.1111/j.1550-7408.1988.tb04141.x.PMID3143826.
- ^Morrison DA, Bornstein S, Thebo P, Wernery U, Kinne J, Mattsson JG (2004). "The current status of the small subunit rRNA phylogeny of the coccidia (Sporozoa)".International Journal for Parasitology.34(4): 501–514.doi:10.1016/j.ijpara.2003.11.006.PMID15013740.
- ^Jirku M, Jirku M, Obornik M, Lukes J, Modry D (2009). "Goussia Labbé, 1896 (Apicomplexa, Eimeriorina) in Amphibia: Diversity, Biology, Molecular Phylogeny and Comments on the Status of the Genus".Protist.160(1): 123–136.doi:10.1016/j.protis.2008.08.003.PMID19038578.
- ^Lom J, Dykova I (1981). "Pathogenicity of some protozoan parasites of cyprinid fishes".Symposia Biologica Hungarica.23:99–118.
- ^Shirley MW (1975). "Enzyme variation inEimeriaspecies of the chicken ".Parasitology.71(3): 369–376.doi:10.1017/s0031182000047144.PMID1202411.S2CID41849277.
- ^Ellis J, Bumstead J (1990). "Eimeriaspecies: studies using rRNA and rDNA probes ".Parasitology.101(1): 1–6.doi:10.1017/s0031182000079671.PMID2235066.S2CID30056695.
- ^Shirley MW (1984)."Eimeria necatrix:Selection and characteristics of a precocious (and attenuated) line ".Avian Pathology.13(4): 657–668.doi:10.1080/03079458408418564.PMID18766877.
- ^Shirley MW (1996). "Eimeria tenella:genetic recombination of markers for precocious development and arprinocid resistance ".Applied Parasitology.37(4): 293–299.PMID9060177.
- ^Procunier JD, Fernando MA, Barta JR (1993). "Species and strain differentiation of Eimeria spp. of the domestic fowl using DNA polymorphisms amplified by arbitrary primers".Parasitology Research.79(2): 98–102.doi:10.1007/bf00932253.PMID8475039.S2CID20235745.
- ^Allen PC, Fetterer RH (2002)."Recent advances in biology and immunobiology ofEimeriaspecies and in diagnosis and control of infection with these coccidian parasites of poultry ".Clinical Microbiology Reviews.15(1): 58–65.doi:10.1128/cmr.15.1.58-65.2002.PMC118059.PMID11781266.
- ^Joyner LP, Norton CC (1978). "The activity of methyl benzoquate and clopidol against Eimeria maxima: synergy and drug resistance".Parasitology.76(3): 369–377.doi:10.1017/s003118200004823x.PMID275786.S2CID23567308.
- ^Shirley MD, et al. (2004). "TheEimeriagenome projects: a sequence of events ".Trends in Parasitology.20(5): 199–201.doi:10.1016/j.pt.2004.02.005.PMID15105014.
- ^Lillehoj HS, Trout JM (1996)."Avian gut-associated lymphoid tissues and intestinal immune responses toEimeriaparasites ".Clinical Microbiology Reviews.9(3): 349–360.doi:10.1128/CMR.9.3.349-360.1996.PMC172898.PMID8809465.
- ^Lindsay DS, et al. (1989). "Specificity and cross-reactivity of hybridoma antibodies generated against Eimeria bovis sporozoites".Veterinary Parasitology.32(2–3): 145–151.doi:10.1016/0304-4017(89)90115-5.PMID2672546.
- ^Chapman HD, et al. (2014). "Absorption and deposition of xanthophylls in broilers challenged with three dosages ofEimeria acervulinaoocysts ".British Poultry Science.55(2): 167–173.doi:10.1080/00071668.2013.879095.PMID24720798.S2CID26935206.
- ^abMaas, J."Coccidiosis in Cattle"(PDF).California Cattlemen's Magazine.Retrieved24 April2014.
- ^Chapman HD, et al. (2002). "Sustainable coccidiosis control in poultry production: the role of live vaccines".International Journal for Parasitology.32(5): 617–629.doi:10.1016/S0020-7519(01)00362-9.PMID11943233.
- ^Ahmad, TA; El-Sayed, BM; El-Sayed, LH (2016)."Development of immunization trials against Eimeria spp".Trials in Vaccinology.5:38–47.doi:10.1016/j.trivac.2016.02.001.ISSN1879-4378.
- ^Smith AL, Hayday AC (1998). "Genetic analysis of the essential components of the immunoprotective response to infection withEimeria vermiformis".International Journal for Parasitology.28(7): 1061–1069.doi:10.1016/S0020-7519(98)00081-2.PMID9724877.
- ^Skirnisson, K.; Thorarinsdottir, S. Th. (May 2007). "Two new Eimeria species (Protozoa: Eimeriidae) from wild rock ptarmigans, Lagopus muta islandorum, in Iceland".Parasitology Research.101(4): 1077–81.doi:10.1007/s00436-007-0589-5.PMID17557155.S2CID21559619.
- ^Yang, Rongchang; Brice, Belinda; Ryan, Una (April 2016)."Morphological and molecular characterization ofEimeria purpureicephalin. sp. (Apicomplexa:Eimeriidae) in a red-capped parrot (Purpureicephalus spurius,Kuhl, 1820) in Western Australia ".International Journal for Parasitology: Parasites and Wildlife.5(1): 34–39.doi:10.1016/j.ijppaw.2016.01.003.PMC4781968.PMID26977403.
