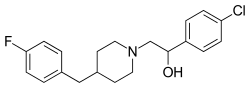
Eliprodil
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.162.249 |
| Chemical and physical data | |
| Formula | C20H23ClFNO |
| Molar mass | 347.86g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eliprodil(codenamedSL-82.0715) is anNMDA antagonistdrug candidate which selectively inhibits the NR2B (GLUN2B) subtype NMDA receptor at submicromolar concentrations. Eliprodil failed a Phase III clinical trial for the treatment of acuteischemic strokein 1996, sponsored by Synthélabo Recherche.[1][2][3]
NMDA receptorsare a key component in mediating glutamate-inducedexcitotoxicity,and it is believed thatNMDA antagonistswould beneuroprotectiveafter a stroke or othertraumatic brain injury.[4]After a traumatic brain injury, neurons become deprived of glucose and oxygen. These neurons quickly lose ATP and become depolarized, which releases glutamate. The extracellular buildup of glutamate triggers the overstimulation ofAMPAand NMDA receptors. This, in turn, causes an influx of Na+and Ca2+.Therefore, when NMDA receptors are activated, there is an increase in intracellular Ca2+concentration. High Ca2+causes fatal metabolic consequences, including neuronal cell death.[3]
References
[edit]- ^The Pharma Letter. 2 December 1996Synthelabo's Eliprodil Fails In Stroke Trials
- ^De Keyser J, Sulter G, Luiten PG (December 1999)."Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing?".Trends in Neurosciences.22(12): 535–40.doi:10.1016/s0166-2236(99)01463-0.PMID10542428.S2CID16302841.
- ^abLee JM, Zipfel GJ, Choi DW (June 1999). "The changing landscape of ischaemic brain injury mechanisms".Nature.399(6738 Suppl): A7-14.doi:10.1038/399a007.PMID10392575.S2CID10086931.
- ^Ikonomidou C, Turski L (October 2002). "Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury?".The Lancet. Neurology.1(6): 383–6.doi:10.1016/s1474-4422(02)00164-3.PMID12849400.S2CID31477519.
