Bioprospecting
Bioprospecting(also known asbiodiversity prospecting) is the exploration of natural sources forsmall molecules,macromoleculesand biochemical and genetic information that could be developed intocommerciallyvaluable products for theagricultural,[2][3]aquaculture,[4][5]bioremediation,[4][6]cosmetics,[7][8]nanotechnology,[4][9]orpharmaceutical[2][10]industries. In the pharmaceutical industry, for example, almost one third of all small-molecule drugs approved by theU.S. Food and Drug Administration(FDA) between 1981 and 2014 were eithernatural productsor compounds derived from natural products.[11]
Terrestrialplants,fungiandactinobacteriahave been the focus of many past bioprospecting programs,[12]but interest is growing in less explored ecosystems (e.g. seas and oceans) and organisms (e.g.myxobacteria,archaea) as a means of identifying new compounds with novelbiological activities.[7][10][13][14]Species may be randomly screened for bioactivity or rationally selected and screened based onecological,ethnobiological,ethnomedical,historicalorgenomicinformation.[10][15][16]
When a region's biological resources orindigenous knowledgeare unethically appropriated or commerciallyexploitedwithout providing fair compensation, this is known asbiopiracy.[12][17]Various international treaties have been negotiated to provide countries legal recourse in the event of biopiracy and to offer commercial actors legal certainty for investment. These include theUNConvention on Biological Diversityand theNagoya Protocol.[2][10]TheWIPOis currentlynegotiating more treatiesto bridge gaps in this field.
Other risks associated with bioprospecting are the overharvesting of individual species and environmental damage, but legislation has been developed to combat these also. Examples include national laws such as the USMarine Mammal Protection Actand USEndangered Species Act,and international treaties such as the UN Convention on Biological Diversity, the UNConvention on the Law of the Sea,theBiodiversity Beyond National Jurisdictions Treaty,and theAntarctic Treaty.[10][18]
Bioprospecting-derived resources and products
[edit]Agriculture
[edit]
Bioprospecting-derived resources and products used in agriculture includebiofertilizers,biopesticidesandveterinary antibiotics.Rhizobiumis a genus of soil bacteria used as biofertilizers,[20]Bacillus thuringiensis(also called Bt) and theannonins(obtained from seeds of the plantAnnona squamosa) are examples of biopesticides,[21][22][19][23]andvalnemulinandtiamulin(discovered and developed from thebasidiomycetefungiOmphalina mutilaandClitopilus passeckerianus) are examples of veterinary antibiotics.[24][25]
Bioremediation
[edit]Examples of bioprospecting products used in bioremediation includeCoriolopsis gallica- andPhanerochaete chrysosporium-derivedlaccaseenzymes, used for treatingbeer factorywastewaterand for dechlorinating and decolorizingpaper milleffluent.[9]
Cosmetics and personal care
[edit]Cosmetics and personal care products obtained from bioprospecting includePorphyridium cruentum-derivedoligosaccharideand oligoelement blends used to treaterythema(rosacea,flushinganddark circles),[7]Xanthobacter autotrophicus-derivedzeaxanthinused forskin hydrationandUVprotection,[8]Clostridium histolyticum-derivedcollagenasesused forskinregeneration,[8]andMicrosporum-derivedkeratinasesused forhair removal.[8]
Nanotechnology and biosensors
[edit]Becausemicrobiallaccaseshave a broadsubstraterange, they can be used inbiosensortechnology to detect a wide range oforganic compounds.For example, laccase-containingelectrodesare used to detectpolyphenolic compoundsinwine,andligninsandphenolsinwastewater.[9]
Pharmaceuticals
[edit]
Many of theantibacterial drugsin current clinical use were discovered through bioprospecting including theaminoglycosides,tetracyclines,amphenicols,polymyxins,cephalosporinsand otherβ-lactam antibiotics,macrolides,pleuromutilins,glycopeptides,rifamycins,lincosamides,streptogramins,andphosphonic acidantibiotics.[10][26]The aminoglycoside antibioticstreptomycin,for example, was discovered from the soil bacteriumStreptomyces griseus,the fusidane antibioticfusidic acidwas discovered from the soil fungusAcremonium fusidioides,and the pleuromutilin antibiotics (eg.lefamulin) were discovered and developed from the basidiomycete fungiOmphalina mutilaandClitopilus passeckerianus.[10][24]
Other examples of bioprospecting-derived anti-infective drugs include theantifungaldruggriseofulvin(discovered from the soil fungusPenicillium griseofulvum),[27]the antifungal andantileishmanialdrugamphotericin B(discovered from the soil bacteriumStreptomyces nodosus),[28]theantimalarialdrugartemisinin(discovered from the plantArtemisia annua),[1][29]and theantihelminthicdrugivermectin(developed from the soil bacteriumStreptomyces avermitilis).[30]
Bioprospecting-derived pharmaceuticals have been developed for the treatment ofnon-communicable diseasesand conditions too. These include theanticancer drugbleomycin(obtained from the soil bacteriumStreptomyces verticillus),[31]theimmunosuppressantdrugciclosporinused to treat autoimmune diseases such asrheumatoid arthritisandpsoriasis(obtained from the soil fungusTolypocladium inflatum),[32]the anti-inflammatory drugcolchicineused to treat and preventgoutflares (obtained from the plantColchicum autumnale),[1]theanalgesicdrugziconotide(developed from thecone snailConus magus),[13]and theacetylcholinesterase inhibitorgalantamineused to treatAlzheimer's disease(obtained from plants in theGalanthusgenus).[33]
Bioprospecting as a discovery strategy
[edit]Bioprospecting has both strengths and weaknesses as a strategy for discovering new genes, molecules, and organisms suitable for development and commercialization.
Strengths
[edit]
Bioprospecting-derivedsmall molecules(also known asnatural products) are more structurally complex than synthetic chemicals, and therefore show greaterspecificitytowardsbiological targets.This is a big advantage indrug discoveryanddevelopment,especiallypharmacologicalaspects of drug discovery and development, where off-target effects can causeadverse drug reactions.[10]
Natural products are also more amenable tomembrane transportthan synthetic compounds. This is advantageous when developingantibacterialdrugs, which may need to traverse both anouter membraneandplasma membraneto reach their target.[10]
For somebiotechnologicalinnovations to work, it is important to haveenzymesthat function at unusually high or low temperatures. An example of this is thepolymerase chain reaction(PCR), which is dependent on aDNA polymerasethat can operate at 60°C and above.[14]In other situations, for exampledephosphorylation,it can be desirable to run the reaction at low temperature.[13]Extremophilebioprospecting is an important source of such enzymes, yielding thermostable enzymes such asTaqpolymerase(fromThermus aquaticus),[14]and cold-adapted enzymes such as shrimpalkaline phosphatase(fromPandalus borealis).[13]
With the Convention on Biological Diversity (CBD) now ratified by most countries, bioprospecting has the potential to bring biodiversity-rich and technologically advanced nations together, and benefit them both educationally and economically (eg. information sharing,technology transfer,new product development,royalty payment).[2][35]
For useful molecules identified throughmicrobialbioprospecting, scale up of production is feasible at reasonable cost because the producing microorganism can beculturedin abioreactor.[8][36]
Weaknesses
[edit]
Although some potentially very useful microorganisms are known to exist in nature (eg.lignocellulose-metabolizing microbes), difficulties have been encountered cultivating these in a laboratory setting.[38]This problem may be resolvable bygenetically manipulatingeasier-to-culture organisms such asEscherichia coliorStreptomyces coelicolorto express thegene clusterresponsible for the desired activity.[14][39]
Isolating and identifying thecompound(s)responsible for a biological extract's activity can be difficult.[39]Also, subsequent elucidation of themechanism of actionof the isolated compound can be time-consuming.[39]Technological advancements inliquid chromatography,mass spectrometryand other techniques are helping to overcome these challenges.[39]
Implementing and enforcing bioprospecting-related treaties and legislation is not always easy.[2][35]Drug development is an inherently expensive and time-consuming process with low success rates, and this makes it difficult to quantify the value of potential products when drafting bioprospecting agreements.[2]Intellectual property rightsmay be difficult to award too. For example, legal rights to amedicinal plantmay be disputable if it has been discovered by different people in different parts of the world at different times.[2]
Whilst the structural complexity of natural products is generally advantageous in drug discovery, it can make the subsequent manufacture of drug candidates difficult. This problem is sometimes resolvable by identifying the part of the natural product structure responsible for activity and developing a simplified synthetic analogue. This was necessary with the natural product halichondrin B, its simplified analogueeribulinnow approved and marketed as ananticancer drug.[40]
Bioprospecting pitfalls
[edit]Errors and oversights can occur at different steps in the bioprospecting process including collection of source material, screening source material forbioactivity,testing isolated compounds fortoxicity,and identification ofmechanism of action.
Collection of source material
[edit]
Prior to collectingbiologicalmaterial ortraditional knowledge,the correct permissions must be obtained from the source country, land owner etc. Failure to do so can result incriminal proceedingsand rejection of any subsequentpatentapplications. It is also important to collect biological material in adequate quantities, to have biological material formallyidentified,and to deposit a voucher specimen with arepositoryfor long-term preservation and storage. This helps ensure any important discoveries are reproducible.[10][13]
Bioactivity and toxicity testing
[edit]When testing extracts and isolated compounds for bioactivity and toxicity, the use ofstandardprotocols (eg.CLSI,ISO,NIH,EURL ECVAM,OECD) is desirable because this improves test result accuracy and reproducibility. Also, if the source material is likely to contain known (previously discovered) active compounds (eg. streptomycin in the case of actinomycetes), then dereplication is necessary to exclude these extracts and compounds from the discovery pipeline as early as possible. In addition, it is important to considersolventeffects on the cells orcell linesbeing tested, to include reference compounds (ie. purechemical compoundsfor which accurate bioactivity and toxicity data are available), to set limits on cell line passage number (eg. 10–20 passages), to include all the necessary positive and negativecontrols,and to be aware of assay limitations. These steps help ensure assay results are accurate, reproducible and interpreted correctly.[10][13]
Identification of mechanism of action
[edit]When attempting to elucidate the mechanism of action of an extract or isolated compound, it is important to use multiple orthogonal assays. Using just a single assay, especially a singlein vitroassay, gives a very incomplete picture of an extract or compound's effect on the human body.[41][42]In the case ofValeriana officinalisroot extract, for example, thesleep-inducingeffects of this extract are due to multiple compounds and mechanisms including interaction withGABA receptorsandrelaxationofsmooth muscle.[41]The mechanism of action of an isolated compound can also be misidentified if a single assay is used because some compoundsinterferewith assays. For example, the sulfhydryl-scavenging assay used to detecthistone acetyltransferaseinhibition can give a false positive result if the test compound reacts covalently with cysteines.[42]
Biopiracy
[edit]The termbiopiracywas coined byPat Mooney,[43]to describe a practice in which indigenous knowledge of nature, originating withindigenous peoples,is used by others for profit, without authorization or compensation to the indigenous people themselves.[44]For example, when bioprospectors draw on indigenous knowledge of medicinal plants which is laterpatentedby medical companies without recognizing the fact that the knowledge is not new or invented by the patenter, this deprives the indigenous community of their potential rights to the commercial product derived from the technology that they themselves had developed.[45]Critics of this practice, such asGreenpeace,[46]claim these practices contribute to inequality between developing countries rich inbiodiversity,and developed countries hostingbiotechfirms.[45]
In the 1990s many large pharmaceutical and drug discovery companies responded to charges of biopiracy by ceasing work on natural products, turning tocombinatorial chemistryto develop novel compounds.[43]
Famous cases of biopiracy
[edit]
The rosy periwinkle
[edit]Therosy periwinklecase dates from the 1950s. The rosy periwinkle, while native toMadagascar,had been widely introduced into other tropical countries around the world well before the discovery ofvincristine.Different countries are reported as having acquired different beliefs about the medical properties of the plant.[47]This meant that researchers could obtain local knowledge from one country and plant samples from another. The use of the plant fordiabeteswas the original stimulus for research. Effectiveness in the treatment of bothHodgkin lymphomaandleukemiawere discovered instead.[48]The Hodgkin lymphoma chemotherapeutic drugvinblastineis derivable from the rosy periwinkle.[49]
The Maya ICBG controversy
[edit]TheMaya ICBG bioprospecting controversytook place in 1999–2000, when theInternational Cooperative Biodiversity Groupled byethnobiologistBrent Berlinwas accused of being engaged in unethical forms of bioprospecting by severalNGOsand indigenous organizations. The ICBG aimed to document the biodiversity ofChiapas,Mexico,and theethnobotanicalknowledge of the indigenousMaya people– in order to ascertain whether there were possibilities of developing medical products based on any of the plants used by the indigenous groups.[50][51]
The Maya ICBG case was among the first to draw attention to the problems of distinguishing between benign forms of bioprospecting and unethical biopiracy, and to the difficulties of securing community participation and prior informed consent for would-be bioprospectors.[52]
The neem tree
[edit]
In 1994, theU.S. Department of AgricultureandW. R. Grace and Companyreceived a European patent on methods of controlling fungal infections in plants using a composition that included extracts from theneemtree (Azadirachta indica), which grows throughoutIndiaandNepal.[53][54][55]In 2000 the patent was successfullyopposedby several groups from the EU and India including the EU Green Party,Vandana Shiva,and theInternational Federation of Organic Agriculture Movements(IFOAM) on the basis that the fungicidal activity of neem extract had long been known inIndian traditional medicine.[55]WR Grace appealed and lost in 2005.[56]
Basmati rice
[edit]In 1997, the US corporationRiceTec(a subsidiary of RiceTec AG of Liechtenstein) attempted to patent certain hybrids ofbasmatirice and semidwarf long-grain rice.[57]The Indian government challenged this patent and, in 2002, fifteen of the patent's twenty claims were invalidated.[58]
The Enola bean
[edit]
The Enola bean is a variety of Mexicanyellow bean,so called after the wife of the man who patented it in 1999.[59]The allegedly distinguishing feature of the variety is seeds of a specific shade of yellow. The patent-holder subsequently sued a large number of importers of Mexican yellow beans with the following result: "...export sales immediately dropped over 90% among importers that had been selling these beans for years, causing economic damage to more than 22,000 farmers in northern Mexico who depended on sales of this bean."[60]A lawsuit was filed on behalf of the farmers and, in 2005, the US-PTO ruled in favor of the farmers. In 2008, the patent was revoked.[61]
Hoodia gordonii
[edit]
Hoodia gordonii,asucculent plant,originates from theKalahari DesertofSouth Africa.For generations it has been known to the traditionally livingSan peopleas anappetite suppressant.In 1996 South Africa'sCouncil for Scientific and Industrial Researchbegan working with companies, includingUnilever,to develop dietary supplements based onHoodia.[62][63][64][65]Originally the San people were not scheduled to receive any benefits from the commercialization of their traditional knowledge, but in 2003 the South African San Council made an agreement with CSIR in which they would receive from 6 to 8% of the revenue from the sale ofHoodiaproducts.[66]
In 2008 after having invested €20 million in R&D onHoodiaas a potential ingredient indietary supplementsfor weight loss, Unilever terminated the project because their clinical studies did not show thatHoodiawas safe and effective enough to bring to market.[67]
Further cases
[edit]The following is a selection of further recent cases of biopiracy. Most of them do not relate to traditional medicines.
- Thirty-six cases of biopiracy in Africa.[68]
- The case of the Maya people'spozoldrink.[69][70]
- The case of the Maya and other people's use ofMimosa tenuifloraand many other cases.[71]
- The case of the Andeanmacaradish.[72]
- The cases ofturmeric(India),[73]karela(India),quinoa(Bolivia),oubliberries (Gabon), and others.[74]
- The case ofcaptopril(developed from a Brazilian tribe'sarrowhead poison).[75]
Legal and political aspects
[edit]This sectionneeds additional citations forverification.(August 2020) |
Patent law
[edit]One common misunderstanding is that pharmaceutical companiespatentthe plants they collect. While obtaining a patent on a naturally occurring organism as previously known or used is not possible, patents may be taken out on specific chemicals isolated or developed from plants. Often these patents are obtained with a stated and researched use of those chemicals.[citation needed]Generally the existence, structure and synthesis of those compounds is not a part of the indigenous medical knowledge that led researchers to analyze the plant in the first place. As a result, even if the indigenous medical knowledge is taken as prior art, that knowledge does not by itself make the active chemical compound "obvious," which is the standard applied under patent law.
In theUnited States,patent lawcan be used to protect "isolated and purified" compounds – even, in one instance, a new chemical element (see USP 3,156,523). In 1873,Louis Pasteurpatented a "yeast" which was "free from disease" (patent #141072). Patents covering biological inventions have been treated similarly. In the 1980 case ofDiamond v. Chakrabarty,theSupreme Courtupheld a patent on a bacterium that had been genetically modified to consume petroleum, reasoning that U.S. law permits patents on "anything under the sun that is made by man." TheUnited States Patent and Trademark Office(USPTO) has observed that "a patent on a gene covers the isolated and purified gene but does not cover the gene as it occurs in nature".[76]
Also possible under US law is patenting acultivar,a new variety of an existing organism. The patent on the Enola bean (now revoked)[77]was an example of this sort of patent. Theintellectual propertylaws of the US also recognizeplant breeders' rightsunder thePlant Variety Protection Act,7 U.S.C. §§ 2321–2582.[78]
Convention on Biological Diversity
[edit]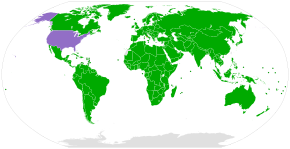
The Convention on Biological Diversity (CBD) came into force in 1993. It secured rights to control access togenetic resourcesfor the countries in which those resources are located. One objective of the CBD is to enable lesser-developed countries to better benefit from their resources and traditional knowledge. Under the rules of the CBD, bioprospectors are required to obtaininformed consentto access such resources, and must share any benefits with the biodiversity-rich country.[80]However, some critics believe that the CBD has failed to establish appropriate regulations to prevent biopiracy.[81]Others claim that the main problem is the failure of national governments to pass appropriate laws implementing the provisions of the CBD.[82]TheNagoya Protocolto the CBD, which came into force in 2014, provides further regulations.[83]The CBD has been ratified, acceded or accepted by 196 countries and jurisdictions globally, with exceptions including theHoly SeeandUnited States.[79]
Bioprospecting contracts
[edit]The requirements for bioprospecting as set by CBD has created a new branch of internationalpatentandtrade law,bioprospecting contracts.[2]Bioprospecting contracts lay down the rules of benefit sharing between researchers and countries, and can bring royalties tolesser-developed countries.However, although these contracts are based on prior informed consent and compensation (unlike biopiracy), every owner or carrier of an indigenous knowledge and resources are not always consulted or compensated,[84]as it would be difficult to ensure every individual is included.[85]Because of this, some have proposed that the indigenous or other communities form a type of representative micro-government that would negotiate with researchers to form contracts in such a way that the community benefits from the arrangements.[85]Unethical bioprospecting contracts (as distinct from ethical ones) can be viewed as a new form of biopiracy.[81]
An example of a bioprospecting contract is the agreement betweenMerckandINBioofCosta Rica.[86]
Traditional knowledge database
[edit]Due to previous cases of biopiracy and to prevent further cases, the Government of India has convertedtraditional Indian medicinalinformation from ancient manuscripts and other resources into an electronic resource; this resulted in theTraditional Knowledge Digital Libraryin 2001.[87]The texts are being recorded fromTamil,Sanskrit,Urdu,PersianandArabic;made available to patent offices in English, German, French, Japanese and Spanish. The aim is to protect India's heritage from being exploited by foreign companies.[88]Hundreds ofyoga posesare also kept in the collection.[88]The library has also signed agreements with leading internationalpatent officessuch asEuropean Patent Office(EPO),United Kingdom Trademark & Patent Office(UKTPO) and theUnited States Patent and Trademark Officeto protecttraditional knowledgefrom biopiracy as it allowspatent examinersat International Patent Offices to access TKDL databases for patent search and examination purposes.[73][89][90]
See also
[edit]- Intellectual capital/Intellectual property
- Natural capital
- Biological patent
- Traditional knowledge/Indigenous knowledge
- Pharmacognosy
- Plant breeders' rights
- Bioethics
- Maya ICBG bioprospecting controversy
- International Cooperative Biodiversity Group
- Biological Diversity Act, 2002
- Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS)(1994)
- International Treaty on Plant Genetic Resources for Food and Agriculture(2001)
References
[edit]- ^abcBuenz EJ, Verpoorte R, Bauer BA (January 2018)."The ethnopharmacologic contribution to bioprospecting natural products".Annual Review of Pharmacology and Toxicology.58(1): 509–530.doi:10.1146/annurev-pharmtox-010617-052703.PMID29077533.
- ^abcdefgh"Mobilizing funding for biodiversity conservation: a user-friendly training guide"(PDF).United Nations.Retrieved17 July2020.
- ^Pandey A, Yarzábal LA (January 2019). "Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments".Applied Microbiology and Biotechnology.103(2): 643–657.doi:10.1007/s00253-018-9515-2.PMID30465306.S2CID53720063.
- ^abcBeattie AJ, Hay M, Magnusson B, de Nys R, Smeathers J, Vincent JF (May 2011)."Ecology and bioprospecting".Austral Ecology.36(3): 341–356.Bibcode:2011AusEc..36..341B.doi:10.1111/j.1442-9993.2010.02170.x.PMC3380369.PMID22737038.
- ^Mazarrasa I, Olsen YS, Mayol E, Marbà N, Duarte CM (October 2014). "Global unbalance in seaweed production, research effort and biotechnology markets".Biotechnology Advances.32(5): 1028–36.doi:10.1016/j.biotechadv.2014.05.002.PMID24858315.
- ^Pascoal F, Magalhães C, Costa R (February 2020)."The link between the ecology of the prokaryotic rare biosphere and its biotechnological potential".Frontiers in Microbiology.11:Article 231.doi:10.3389/fmicb.2020.00231.PMC7042395.PMID32140148.
- ^abcAbida H, Ruchaud S, Rios L, Humeau A, Probert I, De Vargas C, Bach S, Bowler C (November 2013)."Bioprospecting marine plankton".Marine Drugs.11(11): 4594–4611.doi:10.3390/md11114594.PMC3853748.PMID24240981.
- ^abcdeGupta PL, Rajput M, Oza T, Trivedi U, Sanghvi G (August 2019)."Eminence of microbial products in cosmetic industry".Natural Products and Bioprospecting.9(4): 267–278.doi:10.1007/s13659-019-0215-0.PMC6646485.PMID31214881.
- ^abcUpadhyay P, Shrivastava R, Agrawal PK (June 2016)."Bioprospecting and biotechnological applications of fungal laccase".3 Biotech.6(1): Article 15.doi:10.1007/s13205-015-0316-3.PMC4703590.PMID28330085.
- ^abcdefghijklmCushnie TP, Cushnie B, Echeverría J, Fowsantear W, Thammawat S, Dodgson JL, Law S, Clow SM (June 2020)."Bioprospecting for antibacterial drugs: a multidisciplinary perspective on natural product source material, bioassay selection and avoidable pitfalls".Pharmaceutical Research.37(7): Article 125.doi:10.1007/s11095-020-02849-1.PMID32529587.S2CID219590658.
- ^Newman DJ, Cragg GM (March 2016)."Natural products as sources of new drugs from 1981 to 2014".Journal of Natural Products.79(3): 629–661.doi:10.1021/acs.jnatprod.5b01055.PMID26852623.
- ^abCluis C (2013)."Bioprospecting: a new western blockbuster, after the gold rush, the gene rush".The Science Creative Quarterly.No. 8. The Science Creative Quarterly (University of British Columbia).Archivedfrom the original on 2014-04-30.Retrieved2013-11-04.
- ^abcdefSvenson J (May 2012)."MabCent: Arctic marine bioprospecting in Norway".Phytochemistry Reviews.12(3): 567–578.doi:10.1007/s11101-012-9239-3.PMC3777186.PMID24078803.
- ^abcdSysoev M, Grötzinger SW, Renn D, Eppinger J, Rueping M, Karan R (February 2021)."Bioprospecting of novel extremozymes from prokaryotes—the advent of culture-independent methods".Frontiers in Microbiology.12:Article 630013.doi:10.3389/fmicb.2021.630013.PMC7902512.PMID33643258.
- ^Saslis-Lagoudakis CH,Savolainen V,Williamson EM, Forest F, Wagstaff SJ, Baral SR, Watson MF, Pendry CA, Hawkins JA (September 2012)."Phylogenies reveal predictive power of traditional medicine in bioprospecting".Proceedings of the National Academy of Sciences of the United States of America.109(39): 15835–40.Bibcode:2012PNAS..10915835S.doi:10.1073/pnas.1202242109.PMC3465383.PMID22984175.
- ^Baana K, Angwech H, Malinga GM (May 2018)."Ethnobotanical survey of plants used as repellents against housefly,Musca domesticaL. (Diptera: Muscidae) in Budondo Subcounty, Jinja District, Uganda ".Journal of Ethnobiology and Ethnomedicine.14(1): Article 35.doi:10.1186/s13002-018-0235-6.PMC5946462.PMID29747673.
- ^"Biopiracy".merriam-webster.Merriam-Webster. 2020.Retrieved17 July2020.
- ^Benson E (February 2012). "Endangered science: the regulation of research by the U.S. Marine Mammal Protection and Endangered Species Acts".Historical Studies in the Natural Sciences.42(1): 30–61.doi:10.1525/hsns.2012.42.1.30.PMID27652415.
- ^abWani JA, Wali AF, Majid S, Rasool S, Rehman MU, Rashid SM, Ali S, Farooq S, Rasool S, Ahmad A, Qamar W (2020). "Bio-Pesticides: Application and Possible Mechanism of Action". In Bhat RA, Hakeem KR, Dervash MA (eds.).Bioremediation and Biotechnology, Vol 2: Degradation of Pesticides and Heavy Metals.Cham. pp. 97–119.doi:10.1007/978-3-030-40333-1_6.ISBN978-3-030-40332-4.S2CID218939420.
{{cite book}}:CS1 maint: location missing publisher (link) - ^John RP, Tyagi RD, Brar SK, Surampalli RY, Prévost D (September 2011). "Bio-encapsulation of microbial cells for targeted agricultural delivery".Critical Reviews in Biotechnology.31(3): 211–226.doi:10.3109/07388551.2010.513327.PMID20879835.S2CID207467630.
- ^Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV (2003)."Bacillus thuringiensiscrystal proteins that target nematodes ".Proceedings of the National Academy of Sciences of the United States of America.100(5): 2760–5.Bibcode:2003PNAS..100.2760W.doi:10.1073/pnas.0538072100.PMC151414.PMID12598644.
- ^Gard IE, Gonzalez JM, et al. (September 1992)."Strains ofBacillus thuringiensisinsecticidal compositions containing the same US5147640A ".Retrieved2020-07-27.
- ^Moeschler HF, Pfluger W, Wendisch D (August 1987)."Pure annonin and a process for the preparation thereof US 4689232 A".Retrieved2020-07-27.
- ^abKavanagh F, Hervey A, Robbins WJ (September 1951)."Antibiotic substances from basidiomycetes: VIII.Pleurotus multilus(Fr.) Sacc. andPleurotus passeckerianusPilat ".Proceedings of the National Academy of Sciences of the United States of America.37(9): 570–574.Bibcode:1951PNAS...37..570K.doi:10.1073/pnas.37.9.570.PMC1063423.PMID16589015.
- ^de Mattos-Shipley KM, Foster GD, and Bailey AM (June 2017)."Insights into the classical genetics ofClitopilus passeckerianus– the pleuromutilin producing mushroom ".Frontiers in Microbiology.8:Article 1056.doi:10.3389/fmicb.2017.01056.PMC5465285.PMID28649239.
- ^Tilli Tansey;Lois Reynolds, eds. (2000).Post Penicillin Antibiotics: From acceptance to resistance?.Wellcome Witnesses to Contemporary Medicine.History of Modern Biomedicine Research Group.ISBN978-1-84129-012-6.OL12568269M.WikidataQ29581637.
- ^Beekman AM, Barrow RA (2014). "Fungal metabolites as pharmaceuticals".Australian Journal of Chemistry.67(6): 827–843.doi:10.1071/ch13639.
- ^Procópio RE, Silva IR, Martins MK, Azevedo JL, Araújo JM (2012)."Antibiotics produced byStreptomyces".The Brazilian Journal of Infectious Diseases.16(5): 466–71.doi:10.1016/j.bjid.2012.08.014.PMID22975171.
- ^Kano S (May 2014). "Artemisinin-based combination therapies and their introduction in Japan".Kansenshogaku Zasshi.88(3 Suppl 9–10): 18–25.PMID24979951.
- ^Saraiva RG, Dimopoulos G (2020). "Bacterial natural products in the fight against mosquito-transmitted tropical diseases".Natural Product Reports.37(3): 338–354.doi:10.1039/c9np00042a.PMID31544193.S2CID202731385.
- ^"Bleomycin".US National Library of Medicine.Retrieved27 July2020.
- ^Borel JF, Kis ZL, Beveridge T (1995). "The History of the Discovery and Development of Cyclosporine (Sandimmune®)".The Search for Anti-Inflammatory Drugs.Boston, MA. pp. 27–63.doi:10.1007/978-1-4615-9846-6_2.ISBN978-1-4615-9848-0.
{{cite book}}:CS1 maint: location missing publisher (link) - ^Russo P, Frustaci A, Del Bufalo A, Fini M, Cesario A (2013). "Multitarget drugs of plants origin acting on Alzheimer's disease".Current Medicinal Chemistry.20(13): 1686–93.doi:10.2174/0929867311320130008.PMID23410167.
- ^Koliou P, Karavasilis V, Theochari M, Pollack SM, Jones RL, Thway K (February 2018)."Advances in the treatment of soft tissue sarcoma: focus on eribulin".Cancer Management and Research.10:207–216.doi:10.2147/CMAR.S143019.PMC5798537.PMID29440930.
- ^abSandhu HS."Bioprospecting: Pros and Cons"(PDF).Punjab Agricultural University.Retrieved7 July2021.
- ^"Pharmaceutical bioreactor / fermentor".American Pharmaceutical Review.Retrieved7 July2021.
- ^Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir M (2015)."A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone)".ScientificWorldJournal.2015:Article 816364.doi:10.1155/2015/816364.PMC4461790.PMID26106644.
- ^Buckley M, Wall J."Microbial energy conversion"(PDF).American Society for Microbiology.Retrieved7 July2021.
- ^abcdAtanasov AG, Zotchev SB, Dirsch VM, INPST, Supuran CT (January 2021)."Natural products in drug discovery: advances and opportunities".Nature Reviews Drug Discovery.20(3): 200–216.doi:10.1038/s41573-020-00114-z.PMC7841765.PMID33510482.
- ^"Success story: halichondrin B (NSC 609395) E7389 (NSC 707389)".Developmental Therapeutics Program,National Cancer Institute.Archived fromthe originalon 2009-07-10.
- ^abHoughton PJ, Howes MJ, Lee CC, Steventon G (April 2007). "Uses and abuses ofin vitrotests in ethnopharmacology: visualizing an elephant ".Journal of Ethnopharmacology.110(3): 391–400.doi:10.1016/j.jep.2007.01.032.PMID17317057.
- ^abDahlin JL, Nissink JW, Strasser JM, Francis S, Higgins L, Zhou H, Zhang Z, Walters MA (March 2015)."PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS".Journal of Medicinal Chemistry.58(5): 2091–2113.doi:10.1021/jm5019093.PMC4360378.PMID25634295.
- ^abPaterson R, Lima N (2016-12-12).Bioprospecting: success, potential and constraints.Paterson, Russell; Lima, Nelson. Cham, Switzerland.ISBN978-3-319-47935-4.OCLC965904321.
{{cite book}}:CS1 maint: location missing publisher (link) - ^Park C, Allaby M.A dictionary of environment and conservation(3 ed.). [Oxford].ISBN978-0-19-182632-0.OCLC970401188.
- ^abWyatt T (2012)."Biopiracy".Encyclopedia of Transnational Crime & Justice.Thousand Oaks: SAGE Publications, Inc. p. 30.doi:10.4135/9781452218588.n11.ISBN978-1-4129-9077-6.
- ^"Agriculture and Food".Green Peace Australia Pacific: What We Do: Food.Greenpeace.Archivedfrom the original on 2008-09-19.Retrieved2013-11-04.
- ^"A traditional brew leads to cancer cure".Smithsonian Institution: Migrations in history: Medical Technology.Smithsonian Institution.Archivedfrom the original on 2014-06-21.Retrieved2013-11-04.
- ^Hafstein VT (26 July 2004). "The Politics of Origins: Collective Creation Revisited".Journal of American Folklore.117(465): 300–315.doi:10.1353/jaf.2004.0073.S2CID145691975.
- ^Karasov C (December 2001)."Focus: who reaps the benefits of biodiversity?".Environmental Health Perspectives.109(12): A582-7.doi:10.2307/3454734.JSTOR3454734.PMC1240518.PMID11748021.
- ^Hayden C (2003).When Nature Goes Public: The Making and Unmaking of Bioprospecting in Mexico.Princeton University Press. pp. 100–105.ISBN978-0-691-09556-1.Retrieved2013-11-04.
- ^Feinholz-Klip D, Barrios LG, Lucas JC (2009). "The Limitations of Good Intent: Problems of Representation and Informed Consent in the Maya ICBG Project in Chiapas, Mexico". In Wynberg R, Schroeder D, Chennells R (eds.).Indigenous Peoples, Consent and Benefit Sharing.Springer Netherlands. pp. 315–331.doi:10.1007/978-90-481-3123-5_17.ISBN978-90-481-3123-5.
- ^Lavery JV (2007)."Case 1: Community Involvement in Biodiversity Prospecting in Mexico".Ethical Issues in International Biomedical Research: A Casebook.Oxford University Press. pp. 21–43.ISBN978-0-19-517922-4.Retrieved2013-11-04.
- ^"Method for controlling fungi on plants by the aid of a hydrophobic extracted neem oil".google.Retrieved30 April2018.
- ^Karen Hoggan for the BBC. May 11, 2000Neem tree patent revokedArchived2013-12-26 at theWayback Machine
- ^abSheridan C (May 2005). "EPO neem patent revocation revives biopiracy debate".Nature Biotechnology.23(5): 511–12.doi:10.1038/nbt0505-511.PMID15877054.S2CID29690410.
- ^BBC News, March 9, 2005India wins landmark patent battleArchived2011-06-01 at theWayback Machine
- ^"Rice lines bas 867 rt1117 and rt112".google.Archivedfrom the original on 30 April 2018.Retrieved30 April2018.
- ^Mukherjee U (June 2008). "A study of the basmati case (India-US basmati rice dispute): The geographical indication perspective".SSRN.doi:10.2139/ssrn.1143209.S2CID130991379.SSRN1143209.
- ^Pallottini L, Garcia E, Kami J, Barcaccia G, Gepts P (1 May 2004)."The Genetic Anatomy of a Patented Yellow Bean".Crop Science.44(3): 968–977.doi:10.2135/cropsci2004.0968.Archived fromthe originalon 18 April 2005.
- ^Goldberg D (2003)."Jack and the Enola Bean".TED Case Studies Number xxx.Danielle Goldberg. Archived fromthe originalon 2013-11-10.Retrieved2013-11-04.
- ^"US Patent Office rejects company's claim for bean commonly grown by Latin American farmers".American Association for the Advancement of Science (AAAS). April 2008.
- ^Maharaj, VJ, Senabe, JV, Horak RM (2008)."Hoodia,a case study at CSIR. Science real and relevant ".2nd CSIR Biennial Conference, CSIR International Convention Centre Pretoria, 17&18 November 2008:4.hdl:10204/2539.
- ^Wynberg R, Schroeder D, Chennells R (30 September 2009).Indigenous Peoples, Consent and Benefit Sharing: Lessons from the San-Hoodia Case.Springer.ISBN978-90-481-3123-5.Retrieved2013-11-04.
- ^Vermeylen S (2007). "Contextualizing 'Fair' and 'Equitable': The San's Reflections on theHoodiaBenefit-Sharing Agreement ".Local Environment.12(4): 423–436.Bibcode:2007LoEnv..12..423V.doi:10.1080/13549830701495252.S2CID153467522.
- ^Wynberg R (2013-10-13)."Hot air overHoodia".Grain: Publications: Seedling.Grain.Archivedfrom the original on 2013-11-03.Retrieved2013-11-03.
- ^Foster LA (April 2001)."InventingHoodia:Vulnerabilities and Epistemic Citizenship in South Africa "(PDF).UCLA Center for the Study of Women: CSW update.UCLA Center for the Study of Women. Archived fromthe original(PDF)on 2014-04-30.Retrieved2013-11-04.
- ^"Nutrition | Unilever".Archived fromthe originalon 2014-04-13.Retrieved2014-04-10.
- ^"Africa suffers 36 cases of biopiracy".GhanaWeb. Archived fromthe originalon January 24, 2013.Retrieved31 March2006.
- ^"Biopiracy - a new threat to indigenous rights and culture in Mexico"(PDF).Global Exchange. Archived fromthe original(PDF)on October 13, 2005.Retrieved13 October2005.
- ^"Biopiracy: the appropriation of indigenous peoples' cultural knowledge"(PDF).New England Law. Archived fromthe original(PDF)on September 25, 2003.Retrieved27 February2008.
- ^"Of patents & piⓇates".Genetic Resources Action International.Retrieved18 July2020.
- ^"Maca: the dubious aphrodisiac Chinese biopirates took from Peru".Dialogo Chino. 31 October 2019.Retrieved18 July2020.
- ^ab"Know Instances of Patenting on the UES of Medicinal Plants in India".PIB, Ministry of Environment and Forests. May 6, 2010.Archivedfrom the original on May 10, 2010.
- ^"The United Kingdom Select Committee on Environmental Audit 1999; Appendices to the Minutes of Evidence, Appendix 7: Trade Related Intellectual Property Rights (TRIPs) and Farmers' Rights".parliament.uk.Retrieved18 July2020.
- ^Ellsworth B (December 2010)."Brazil to step up crackdown on" biopiracy "".Ruters.Archivedfrom the original on 7 September 2012.Retrieved18 July2020.
- ^"Department of Commerce: United States Patent and Trademark Office [Docket No. 991027289-0263-02] RIN"(PDF),Federal Register: Notices,vol. 66, no. 4, Office of the Federal Register of the National Archives and Records Administration, pp. 1092–1099, 2001-01-05,archived(PDF)from the original on 2013-02-24,retrieved2013-11-04
- ^Crouch D (July 2009)."Mexican yellow bean patent finally cooked".PatentlyO.Retrieved27 July2020.
- ^Chen JM (2006). "The Parable of the Seeds: Interpreting the Plant Variety Protection Act in Furtherance of Innovation Policy".Notre Dame Law Review.81(4): 105–166.SSRN784189.
- ^abcd"Convention on Biological Diversity: List of parties".United Nations Secretariat of the Convention on Biological Diversity.April 2011.Retrieved2020-08-03.
- ^Notman N (August 2012)."Cracking down on wildlife trafficking".Image.Archived fromthe originalon 12 August 2014.
CBD stating that the benefits arising from the use of genetic resources should be shared in a fair and equitable way (Rau, 2010)
- ^abFinegold DL, Bensimon CM, Daar AS, Eaton ML, Godard B, Knoppers BM, Mackie J, Singer PA (July 2005). "Conclusion: Lessons for Companies and Future Issues".BioIndustry Ethics.Elsevier. pp. 331–354.doi:10.1016/b978-012369370-9/50036-7.ISBN978-0-12-369370-9.
- ^"Policy Commissions".International Chamber of Commerce: About ICC.International Chamber of Commerce.Archivedfrom the original on 2013-11-02.Retrieved2013-11-03.
- ^"The Nagoya Protocol on Access and Benefit-sharing".United Nations Secretariat of the Convention on Biological Diversity.July 2020.Retrieved2020-08-01.
- ^Shiva V (2007). "Bioprospecting as Sophisticated Biopiracy".Signs: Journal of Women in Culture and Society.32(2): 307–313.doi:10.1086/508502.ISSN0097-9740.S2CID144229002.
- ^abMillum J (2010)."How Should the Benefits of Bioprospecting Be Shared?".Hastings Center Report.40(1): 24–33.doi:10.1353/hcr.0.0227.ISSN1552-146X.PMC4714751.PMID20169653.
- ^Eberlee J (2000-01-21)."Assessing the Benefits of Bioprospecting in Latin America"(PDF).IDRC Reports Online.IDRC. Archived fromthe original(PDF)on 2013-06-23.Retrieved2013-11-03.
- ^Bisht TS, Sharma SK, Sati RC, Rao VK, Yadav VK, Dixit AK, Sharma AK, Chopra CS (March 2015)."Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes".Journal of Food Science and Technology.52(3): 1543–51.doi:10.1007/s13197-013-1155-z.PMC4348260.PMID25745223.
- ^ab"India hits back in 'bio-piracy' battle".2005-12-07.Retrieved2019-04-11.
- ^Koshy JP (2010-04-28)."CSIR wing objects to Avesthagen patent claim".Companies.Live Mint.Archivedfrom the original on 2010-04-30.Retrieved2013-11-04.
- ^"India Partners with US and UK to Protect Its Traditional Knowledge and Prevent Bio-Piracy"(Press release). Press Information Bureau, Ministry of Health and Family Welfare, Government of India. 2010-04-28.Archivedfrom the original on 2013-05-31.Retrieved2013-11-04.
Bibliography and resources
[edit]- The Secretariat of the Convention on Biological Diversity (United Nations Environment Programme) maintains aninformation centrewhich as of April 2006 lists some 3000 "monographs, reports and serials".
- Secretariat of the Convention on Biological Diversity (United Nations Environment Programme),Bibliography of Journal Articles on the Convention on Biological Diversity(March 2006). Contains references to almost 200 articles. Some of these are available in full text from theCBD information centre.
- Shiva V (1997).Biopiracy: The Plunder of Nature and Knowledge.South End Press.
- Chen J(2005). "Biodiversity and Biotechnology: A Misunderstood Relation".Michigan State Law Review.2005:51–102.SSRN782184.
External links
[edit]- Out of Africa: Mysteries of Access and Benefit-Sharing– a 2006 report on biopiracy in Africa byThe Edmonds Institute
- Cape Town Declaration– Biowatch South Africa
- Genetic Resources Action International (GRAIN)
- Indian scientist denies accusation of biopiracy–SciDev.Net
- African 'biopiracy' debate heats up–SciDev.Net
- Bioprospecting: legitimate research or 'biopiracy'?–SciDev.Net
- ETC Group papers on Biopiracy:Topics include: Monsanto's species-wide patent on all genetically modified soybeans (EP0301749); Synthetic Biology Patents (artificial, unique life forms); Terminator Seed Technology; etc...
- Who Owns Biodiversity, and How Should the Owners Be Compensated?,Plant Physiology,April 2004, Vol. 134, pp. 1295–1307
- Heald PJ (2001). "'Your Friend in the Rain Forest': An Essay on the Rhetoric of Biopiracy ".SSRN Electronic Journal.doi:10.2139/ssrn.285177.



