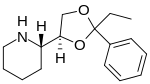
Etoxadrol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etoxadrol(CL-1848C) is adissociativeanaestheticdrug that has been found to be anNMDA antagonistand produce similar effects toPCPin animals.[1][2]Etoxadrol, along with another related drugdexoxadrol,were developed asanalgesicsfor use in humans, but development was discontinued in the late 1970s after patients reported side effects such asnightmaresandhallucinations.[3][4][5]
Chemical structure
[edit]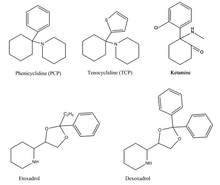
Phenicyclidine (PCP),tenocyclidine (TCP),etoxadrol and its precursor,dexoxadrolhave related chemical structures.[6]These drugs all act similarly on thenervous system,acting asdissociativehallucinogens(meaning that they interfere with normal sensory signals, replacing them withhallucinationsof any sensory modality) withanestheticandanalgesicproperties.
Pharmacodynamics
[edit]Etoxadrol is anon-competitiveNMDA receptor antagonist.[7]It binds with highaffinityto thePCPbinding siteon theNMDA receptor(Ki= 107 nM, determined by the displacement ofradiolabeledTCP).[1][3]Normally, the inactivatedNMDA receptorpossesses amagnesium (Mg2+)block in thechannel,blocking the passage ofcations.[8]
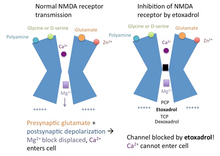
When theneurotransmitterglutamatebinds to theNMDA receptor,and thepostsynapticcell membraneisdepolarized(from the postsynaptic cell being activated), themagnesiumblock in theNMDA receptorchannel is displaced.Calcium (Ca2+)andsodium (Na+)can enter thecellvia the open channel, whilepotassium (K+)can exit the cell. EtoxadrolantagonizestheNMDA receptorby binding to thePCPsite, located just above themagnesiumblock in theion channel.In the event that themagnesiumblock is displaced, etoxadrol blocks theNMDA receptorchannel, preventingcationsfrom entering or exiting the channel. This mechanism of action also applies toPCP,TCP,ketamineanddexoxadrol.
Etoxadrol binding does not affect thebinding affinityof other sites on theNMDA receptor,as found by binding studies showing the displacement ofradiolabeledTCPby etoxadrol (TCP binding in the absence of etoxadrol:Ki= 19.2 x 10−9M, Bmax= 1.36 pmol/mgprotein;TCP binding in the presence of etoxadrol: Ki= 21.7 x 10−9M, Bmax=.66 pmol/mg protein).[9]
Despite itsanestheticandanalgesiceffects, etoxadrol does not interact withbenzodiazepine,muscarinic acetylcholine,ormu opioid receptors.[9]However, etoxadrol may act in thedopaminereward pathway,explaining itsreinforcing properties.[6]
Pharmacokinetics
[edit]Etoxadrol goes into effect 90 seconds afterintravenous (IV) administration,and itsanestheticeffects typically last for half an hour to an hour.[5][10]Since etoxadrol is administered intravenously, thebioavailable doseis always the same as the administered dose. Etoxadrol'sanalgesiceffects can last for up to 2 hours or more after patients have regainedconsciousness.[11]
Etoxadrol islipophilicand can readily cross theblood–brain barrier.Because of itslipophilicstructure, etoxadrol can be absorbed byfat tissuesandorgans(e.g. theliver). Etoxadrol also acts on therespiratoryandcardiovascular systems.[10]
Treatment
[edit]Etoxadrol was intended as ananestheticfor patients requiring particularly long periods ofanesthesiaforsurgery.As ananesthetic,etoxadrol is morepotentthanketamine,but less potent thanPCP.[11]
Etoxadrol is also a potentanalgesic.Patients given etoxadrol often reported that they were aware of experiencingpainupon waking fromanesthesia,but it did not bother them.[5]Post-operative analgesics are rarely required after patients undergoingsurgeryare administered etoxadrol.
Etoxadrol (along withketamine,dexoxadrol,and otherPCP-like drugs) is ananticonvulsant,preventingtonic seizuresinmicethat are administeredpentylenetetrazol (PTZ),which normally inducesseizures.[12]
Side effects
[edit]Likeketamine,etoxadrol produces increases inheart rateandrespiratory rate.[10]Etoxadrol may also causevomiting.[5]At high enoughdoses,etoxadrol also exhibits effects on themuscular systemsuch asconvulsionsor loss of therighting reflex.[13]When administered in excess, etoxadrol can belethalon therespiratory system.Monkeysgiven extremely high (> 20 mg/kg)dosesof etoxadrol died of apparentrespiratory failure.
Etoxadrol produces a wide variety ofdreams,ranging from pleasant to frightening or aversive.[11]Approximately half of patients given etoxadrol report pleasant dreams, 25% report unpleasant dreams, and the remaining 25% experience no dreams at all. Such dreams were frequently described as “floating,” “puffy” or “out of this world." Dreams andhallucinationsmay persist for as long as 18 to 24 hours. In rare cases, etoxadrol can induce periods ofpsychotic activityduring this recovery period.[5]
In thebrain,etoxadrol slows down thesynthesisofserotoninto 50-60% of control rates and speeds up the rate ofdopaminesynthesis by up to 200% of the normal rate 4–6 hours afterintravenous administration.[5]
Like a number of otherdrugs(e.g.cocaine), etoxadrol has been found to exhibitreinforcing properties.Monkeyswill self-administer etoxadrol,dexoxadrolorPCPin a lever-pressing paradigm.[6]
References
[edit]- ^abThurkauf A, Zenk PC, Balster RL, May EL, George C, Carroll FI, et al. (December 1988). "Synthesis, absolute configuration, and molecular modeling study of etoxadrol, a potent phencyclidine-like agonist".Journal of Medicinal Chemistry.31(12): 2257–63.doi:10.1021/jm00120a004.PMID2903930.
- ^Thurkauf A, Mattson MV, Richardson S, Mirsadeghi S, Ornstein PL, Harrison EA, et al. (April 1992). "Analogues of the dioxolanes dexoxadrol and etoxadrol as potential phencyclidine-like agents. Synthesis and structure-activity relationships".Journal of Medicinal Chemistry.35(8): 1323–9.doi:10.1021/jm00086a001.PMID1349351.
- ^abSax M, Wünsch B (2006). "Relationships between the structure of dexoxadrol and etoxadrol analogues and their NMDA receptor affinity".Current Topics in Medicinal Chemistry.6(7): 723–32.doi:10.2174/156802606776894483.PMID16719812.
- ^Aepkers M, Wünsch B (December 2005). "Structure-affinity relationship studies of non-competitive NMDA receptor antagonists derived from dexoxadrol and etoxadrol".Bioorganic & Medicinal Chemistry.13(24): 6836–49.doi:10.1016/j.bmc.2005.07.030.PMID16169732.
- ^abcdefFrederickson EL, Longnecker DE, Allen GW (May–Jun 1976). "Clinical investigation of a new intravenous anesthetic--etoxadrol hydrochloride (CL-1848; U-37862A)".Anesthesia and Analgesia.55(3): 335–9.doi:10.1213/00000539-197605000-00010.PMID5921.S2CID45801472.
- ^abcBrady KT, Woolverton WL, Balster RL (January 1982). "Discriminative stimulus and reinforcing properties of etoxadrol and dexoxadrol in monkeys".The Journal of Pharmacology and Experimental Therapeutics.220(1): 56–62.PMID6118431.
- ^Domino EF (January 1992)."Chemical dissociation of human awareness: focus on non-competitive NMDA receptor antagonists"(PDF).Journal of Psychopharmacology.6(3): 418–24.doi:10.1177/026988119200600312.hdl:2027.42/68872.PMID22291389.S2CID17738916.
- ^Paradiso MF, Bear BW, Connors MA (2007).Neuroscience: exploring the brain(3rd ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp.154–155.ISBN978-0781760034.
- ^abThurkauf A, Mattson MV, Huguenin PN, Rice KC, Jacobson AE (October 1988). "Etoxadrol-meta-isothiocyanate: a potent, enantioselective, electrophilic affinity ligand for the phencyclidine-binding site".FEBS Letters.238(2): 369–74.doi:10.1016/0014-5793(88)80514-3.PMID2901991.S2CID22308090.
- ^abcTraber DL, Priano LL, Wilson RD (November 1970). "Effects of CL 1848C, a new dissociative anesthetic, on the canine cardiovascular and respiratory systems".The Journal of Pharmacology and Experimental Therapeutics.175(2): 395–403.PMID5481707.
- ^abcWilson RD, Traber DL, Barratt E, Creson DL, Schmitt RC, Allen CR (Mar–Apr 1970)."Evaluation of CL-1848C: a new dissociative anesthetic in normal human volunteers".Anesthesia and Analgesia.49(2): 236–41.doi:10.1213/00000539-197003000-00011.PMID4931158.S2CID33036876.
- ^Hayes BA, Balster RL (October 1985). "Anticonvulsant properties of phencyclidine-like drugs in mice".European Journal of Pharmacology.117(1): 121–5.doi:10.1016/0014-2999(85)90480-7.PMID4085541.
- ^Hidalgo J, Dileo RM, Rikimaru MT, Guzman RJ, Thompson CR (Mar–Apr 1971). "Etoxadrol (CL-1848C) a new dissociative anesthetic: studies in primates and other species".Anesthesia and Analgesia.50(2): 231–9.doi:10.1213/00000539-197103000-00016.PMID4994714.S2CID29976263.
