Exocytosis
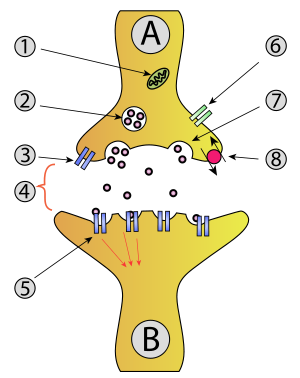
- Mitochondrion
- Synaptic vesiclewithneurotransmitters
- Autoreceptor
- Synapsewith neurotransmitter released (serotonin)
- Postsynaptic receptors activated by neurotransmitter (induction of a postsynaptic potential)
- Calcium channel
- Exocytosis of a vesicle
- Recaptured neurotransmitter
Exocytosis(/ˌɛksoʊsaɪˈtoʊsɪs/[1][2]) is a form ofactive transportandbulk transportin which a cell transportsmolecules(e.g.,neurotransmittersandproteins) out of the cell (exo-+cytosis). As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart,endocytosis,are used by all cells because mostchemical substancesimportant to them are largepolarmolecules that cannot pass through thehydrophobicportion of thecell membranebypassivemeans. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane calledporosomes.Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
In exocytosis, membrane-bound secretoryvesiclesare carried to thecell membrane,where they dock and fuse atporosomesand their contents (i.e., water-soluble molecules) are secreted into the extracellular environment. Thissecretionis possible because the vesicle transientlyfuseswith the plasma membrane. In the context ofneurotransmission,neurotransmitters are typically released fromsynaptic vesiclesinto thesynaptic cleftvia exocytosis; however, neurotransmitters can also be released viareverse transportthroughmembrane transport proteins.
Exocytosis is also a mechanism by which cells are able to insertmembrane proteins(such asion channelsandcell surface receptors),lipids,and other components into the cell membrane. Vesicles containing these membrane components fully fuse with and become part of the outer cell membrane.
History[edit]
The term was proposed byDe Duvein 1963.[3]
Types[edit]
Ineukaryotes,there are two types of exocytosis: 1)Ca2+triggered non-constitutive (i.e., regulated exocytosis) and 2) non-Ca2+triggered constitutive (i.e., non-regulated).
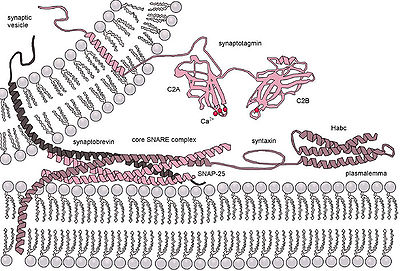
Ca2+triggered non-constitutiveexocytosis requires an external signal, a specific sorting signal on the vesicles, aclathrincoat, as well as an increase in intracellular calcium. In multicellular organisms, this mechanism initiates many forms of intercellular communication such as synaptic transmission, hormone secretion by neuroendocrine cells, and immune cells secretion. In neurons and endocrine cells, the SNARE-proteins and SM-proteins catalyze the fusion by forming a complex that brings the two fusion membranes together. For instance, in synapses, the SNARE complex is formed bysyntaxin-1andSNAP25at the plasma membrane andVAMP2at the vesicle membrane.[4]Exocytosis in neuronalchemical synapsesis Ca2+triggered and serves interneuronal signalling. The calcium sensors that triggers exocytosis might interact either with the SNARE complex or with the phospholipids of the fusing membranes. Synaptotagmin has been recognized as the major sensor for Ca2+triggered exocytosis in animals.[5]However, synaptotagmin proteins are absent in plants and unicellular eukaryotes. Other potential calcium sensors for exocytosis are EF-hand proteins (Ex: Calmodulin) and C2-domain (Ex: Ferlins, E-synaptotagmin, Doc2b) containing proteins. It is unclear how the different calcium sensors can cooperate together and mediate the calcium triggered kinetics of exocytosis in a specific fashion.[6]
Constitutive exocytosisis performed by all cells and serves the release of components of theextracellular matrixor delivery of newly synthesized membrane proteins that are incorporated in theplasma membraneafter the fusion of the transportvesicle.There is no clear consensus about the machinery and molecular processes that drive the formation, budding, translocation and fusion of the post-Golgi vesicles to the plasma membrane. The fusion involves membrane tethering (recognition) and membrane fusion. It is still unclear if the machinery between the constitutive and regulated secretion is different. The machinery required for constitutive exocytosis has not been studying as much as the mechanism of regulated exocytosis. Two tethering complexes are associated with constitutive exocytosis in mammals, ELKS and Exocyst. ELKS is a large coiled-coil protein, also involved in synaptic exocytosis, marking the 'hotspots' fusion points of the secretory carriers fusion. Exocyst is an octameric protein complex. In mammals, exocyst components localize in both plasma membrane, and Golgi apparatus and the exocyst proteins are colocalized at the fusion point of the post-Golgi vesicles. The membrane fusion of the constitutive exocytosis, probably, is mediated by SNAP29 and Syntaxin19 at the plasma membrane and YKT6 or VAMP3 at the vesicle membrane.[7]
Vesicular exocytosisinprokaryotegram negative bacteriais a third mechanism and latest finding in exocytosis. The periplasm is pinched off asbacterial outer membrane vesicles(OMVs) for translocating microbial biochemical signals intoeukaryotichost cells[8]or other microbes located nearby,[9]accomplishing control of the secreting microbe on its environment - including invasion of host, endotoxemia, competing with other microbes for nutrition, etc. This finding ofmembrane vesicle traffickingoccurring at thehost–pathogen interfacealso dispels the myth that exocytosis is purely a eukaryotic cell phenomenon.[10]
Steps[edit]
Five steps are involved in exocytosis:
Vesicle trafficking[edit]
Certain vesicle-trafficking steps require the transportation of a vesicle over a moderately small distance. For example, vesicles that transport proteins from theGolgi apparatusto the cell surface area, will be likely to use motor proteins and a cytoskeletal track to get closer to their target. Before tethering would have been appropriate, many of the proteins used for the active transport would have been instead set for passive transport, because the Golgi apparatus does not require ATP to transport proteins. Both the actin- and the microtubule-base are implicated in these processes, along with severalmotor proteins.Once the vesicles reach their targets, they come into contact with tethering factors that can restrain them.
Vesicle tethering[edit]
It is useful to distinguish between the initial, loosetetheringof vesicles to their objective from the more stable,packinginteractions. Tethering involves links over distances of more than about half the diameter of a vesicle from a given membrane surface (>25 nm). Tethering interactions are likely to be involved in concentrating synaptic vesicles at thesynapse.
Vesicle docking[edit]
Secretory vesicles transiently dock and fuse at theporosomeat the cell plasma membrane, via a tight t-/v-SNARE ring complex.
Vesicle priming[edit]
In neuronal exocytosis, the termpriminghas been used to include all of the molecular rearrangements and ATP-dependent protein and lipid modifications that take place after initial docking of a synaptic vesicle but before exocytosis, such that the influx of calcium ions is all that is needed to trigger nearly instantaneousneurotransmitterrelease. In other cell types, whose secretion is constitutive (i.e. continuous, calcium ion independent, non-triggered) there is no priming.
Vesicle fusion[edit]

Transient vesicle fusion is driven bySNAREproteins, resulting in release of vesicle contents into the extracellular space (or in case of neurons in the synaptic cleft).
The merging of the donor and the acceptor membranes accomplishes three tasks:
- The surface of the plasma membrane increases (by the surface of the fused vesicle). This is important for the regulation of cell size, e.g., during cell growth.
- The substances within the vesicle are released into the exterior. These might be waste products ortoxins,or signaling molecules likehormonesorneurotransmittersduringsynaptic transmission.
- Proteinsembedded in the vesicle membrane are now part of the plasma membrane. The side of the protein that was facing theinsideof the vesicle now faces theoutsideof the cell. This mechanism is important for the regulation of transmembrane and transporters.
Vesicle retrieval[edit]
Retrieval of synaptic vesicles occurs byendocytosis.Most synaptic vesicles are recycled without a full fusion into the membrane (kiss-and-run fusion) viaporosome.Non-constitutive exocytosis and subsequentendocytosisare highly energy expending processes, and thus, are dependent onmitochondria.[12]
Examination of cells following secretion using electron microscopy demonstrate increased presence of partially empty vesicles following secretion. This suggested that during the secretory process, only a portion of the vesicular content is able to exit the cell. This could only be possible if the vesicle were to temporarily establish continuity with the cell plasma membrane atporosomes,expel a portion of its contents, then detach, reseal, and withdraw into the cytosol (endocytose). In this way, the secretory vesicle could be reused for subsequent rounds of exo-endocytosis, until completely empty of its contents.[13]
See also[edit]
- Endocytosis
- Pinocytosis
- Phagocytosis
- Membrane nanotube
- Viral shedding
- Presynaptic active zone
- Residual body
- Degranulation
References[edit]
- ^"Exocytosis".LexicoUK English Dictionary.Oxford University Press.Archived fromthe originalon 2020-03-22.
- ^"Exocytosis".Merriam-Webster Dictionary.Retrieved2016-01-21.
- ^Rieger, Rigomar; Michaelis, Arnd; Green, Melvin M. (2012-12-06).Glossary of Genetics: Classical and Molecular.Springer Science & Business Media.ISBN978-3-642-75333-6.
- ^Shin, O. H. (2011-01-17). Terjung, Ronald (ed.).Comprehensive Physiology.Vol. 4 (1 ed.). Wiley. pp. 149–175.doi:10.1002/cphy.c130021.ISBN978-0-470-65071-4.PMID24692137.
- ^Wolfes, Anne C; Dean, Camin (August 2020)."The diversity of synaptotagmin isoforms".Current Opinion in Neurobiology.63:198–209.doi:10.1016/j.conb.2020.04.006.PMID32663762.S2CID220480746.
- ^Pang, Zhiping P; Südhof, Thomas C (August 2010)."Cell biology of Ca2+-triggered exocytosis".Current Opinion in Cell Biology.22(4): 496–505.doi:10.1016/j.ceb.2010.05.001.PMC2963628.PMID20561775.
- ^Stalder, Danièle; Gershlick, David C. (November 2020)."Direct trafficking pathways from the Golgi apparatus to the plasma membrane".Seminars in Cell & Developmental Biology.107:112–125.doi:10.1016/j.semcdb.2020.04.001.PMC7152905.PMID32317144.
- ^YashRoy R C (1993) Electron microscope studies of surface pili and vesicles ofSalmonella3,10:r:- organisms.Indian Journal of Animal Sciences,vol. 63, pp. 99-102.https:// researchgate.net/publication/230817087_Electron_microscope_studies_of_surface_pilli_and_vesicles_of_Salmonella_310r-_organisms?ev=prf_pub
- ^Kadurugamuwa, J L; Beveridge, T J (1996)."Bacteriolytic effect of membrane vesicles fromPseudomonas aeruginosaon other bacterial including pathogens: conceptually new antibiotics ".Journal of Bacteriology.178(10): 2767–2774.doi:10.1128/jb.178.10.2767-2774.1996.PMC178010.PMID8631663.
- ^YashRoy, R.C. (1998)."Discovery of vesicular exocytosis in procaryotes and its role in Salmonella invasion"(PDF).Current Science.75(10): 1062–1066.
- ^Georgiev, Danko D.; James F. Glazebrook (2007). "Subneuronal processing of information by solitary waves and stochastic processes". In Lyshevski, Sergey Edward (ed.).Nano and Molecular Electronics Handbook.Nano and Microengineering Series. CRC Press. pp. 17–1–17–41.doi:10.1201/9781315221670-17.ISBN978-0-8493-8528-5.S2CID199021983.
- ^Ivannikov, M.; et al. (2013)."Synaptic vesicle exocytosis in hippocampal synaptosomes correlates directly with total mitochondrial volume".J. Mol. Neurosci.49(1): 223–230.doi:10.1007/s12031-012-9848-8.PMC3488359.PMID22772899.
- ^Boron, WF & Boulpaep, EL (2012),Medical Physiology. A Cellular and Molecular Approach,vol. 2, Philadelphia: Elsevier[permanent dead link]
External links[edit]
- Exocytosisat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
