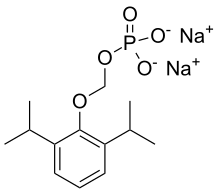
Fospropofol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs | Monograph |
| License data | |
| Pregnancy category |
|
| Dependence liability | unknown |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Protein binding | 98%[1] |
| Metabolism | Hepaticglucuronidation |
| Eliminationhalf-life | 0.81 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C13H21O5P |
| Molar mass | 288.280g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fospropofol(INN[3]), often used as the disodium salt (trade nameLusedra[4]) is anintravenoussedative-hypnoticagent. It is currently approved for use in sedation of adult patients undergoingdiagnosticortherapeuticprocedures such asendoscopy.
Clinical applications[edit]
Severalwater-solublederivatives andprodrugsof the widely used intravenous anesthetic agentpropofolhave been developed, of which fospropofol has been found to be the most suitable for clinical development thus far.[5][6]Purported advantages of this water-solublechemical compoundinclude less pain at the site of intravenous administration, less potential forhyperlipidemiawith long-term administration, and less chance forbacteremia.[citation needed]Often, fospropofol is administered in conjunction with an opioid such as fentanyl.[citation needed]
Clinical pharmacology[edit]
Mechanism of action[edit]
Fospropofol is a prodrug of propofol; as anorganophosphateit is metabolized byalkaline phosphatasestophosphateandformaldehydeand theactive metabolite,propofol.
Pharmacodynamics[edit]
Pharmacokinetics[edit]
Initial trial results on fospropofol pharmacokinetics were retracted by the investigators. As of 2011, new results were not available.[7]
Controlled substance[edit]
Fospropofol is classified as aSchedule IV controlled substancein the United States'Controlled Substances Act.[8]
See also[edit]
References[edit]
- ^ab"LUSEDRA (fospropofol disodium) Injection"(PDF).Woodcliff Lake, New Jersey: Eisai Inc. October 2009. Archived fromthe original(PDF)on 22 November 2010.Retrieved2 August2010.
- ^"Fospropofol disodium".PubChem Compound.Bethesda, Maryland: U.S. National Library of Medicine.Retrieved9 February2017.
- ^"Recommended INNs 2006, pt 56"(PDF).World Health Organization.Retrieved20 April2016.
- ^"FDA Approves Fospropofol and Follows ASAs Labeling Recommendation".American Society of Anesthesiologists. 2008-12-15. Archived fromthe originalon 2011-05-26.Retrieved2011-03-30.
- ^Cooke A, Anderson A, Buchanan K, Byford A, Gemmell D, Hamilton N, et al. (April 2001). "Water-soluble propofol analogues with intravenous anaesthetic activity".Bioorganic & Medicinal Chemistry Letters.11(7): 927–930.doi:10.1016/S0960-894X(01)00088-9.PMID11294393.
- ^Bennett DJ, Anderson A, Buchanan K, Byford A, Cooke A, Gemmell DK, et al. (June 2003). "Novel water soluble 2,6-dimethoxyphenyl ester derivatives with intravenous anaesthetic activity".Bioorganic & Medicinal Chemistry Letters.13(12): 1971–1975.doi:10.1016/S0960-894X(03)00346-9.PMID12781176.
- ^Mahajan B, Kaushal S, Mahajan R (January 2012)."Fospropofol: pharmacokinetics?".Journal of Anaesthesiology Clinical Pharmacology.28(1): 134–135.doi:10.4103/0970-9185.92472.PMC3275955.PMID22345970.
- ^"Schedule of Controlled Substances; Placement of Fospropofol into Schedule IV[permanent dead link],"74 Federal Register 192 (October 6, 2009), pp. 51234–51236.
