Gallium halides
There are three sets ofgallium halides,the trihalides wheregalliumhasoxidation state+3, the intermediate halides containing gallium inoxidation states+1, +2 and +3 and some unstable monohalides, where gallium hasoxidation state+1.
Trihalides
[edit]All four trihalides are known. They all contain gallium in the +3 oxidation state. Their proper names are gallium(III) fluoride, gallium(III) chloride, gallium(III) bromide and gallium(III) iodide.
- GaF3
- GaF3is a white solid which sublimes before it melts, with an estimated melting point above 1000 °C. It contains 6 co-ordinate gallium atoms with a three-dimensional network of GaF6octahedrasharing common corners.
- GaCl3,GaBr3andGaI3
- These all have lower melting points than GaF3,(GaCl3mp 78 °C, GaBr3mp 122 °C, GaI3mp 212 °C) reflecting the fact that their structures all contain dimers with 4 coordinate gallium atoms and 2 bridging halogen atoms. Thus, this halides have molecular formula Ga2Cl6,Ga2Br6,Ga2I6,respectively. They are allLewis acids,forming mainly 4 co-ordinate adducts.GaCl3is the most commonly used trihalide.
Intermediate halides
[edit]Intermediate chlorides, bromides and iodides exist. They contain gallium in oxidation states +1, +2 and +3.
- Ga3Cl7
- This compound contains the Ga2Cl7−ion, which has a structure similar to thedichromate,Cr2O72−,ion with two tetrahedrally coordinated gallium atoms sharing a corner. The compound can be formulated as gallium(I) heptachlorodigallate(III), GaIGaIII2Cl7.[1]
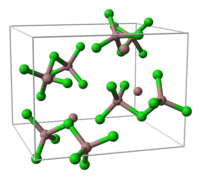


unit cellof Ga3Cl7 part of thecrystal structure structure of [Ga2Cl7]−
- GaCl2,GaBr2and GaI2
- These are the best known and most studied intermediate halides. They contain gallium in oxidation states +1 and +3 and are formulated GaIGaIIIX4.The dihalides are unstable in the presence of waterdisproportionatingto gallium metal and gallium(III) entities. They are soluble in aromatic solvents, where arene complexes have been isolated and the arene isη6coordinated to the Ga+ion. With some ligands, L, e.g. dioxane, a neutral complex, Ga2X2L2,with a gallium-gallium bond is produced. These compounds have been used as a route into gallium chain and cluster compounds.[2]
- Ga2Br3and Ga2I3
- These are formulated GaI2GaII2Br6and GaI2GaII2I6respectively. Both anions contain a gallium-gallium bond where gallium has a formal oxidation state of +2. The Ga2Br62−anion is eclipsed like the In2Br62−anion in In2Br3whereas the Ga2I62−anion is isostructural with Si2Cl6with a staggered conformation.
Monohalides
[edit]None of the monohalides are stable at room temperature. The previously reported GaBr and GaI produced from fusing gallium with the trihalide have been shown to be mixtures of metallic gallium with, respectively, Ga2Br3and Ga2I3.
- GaCl and GaBr
- GaCl and GaBr have been produced in the gas form from the reaction of HX and molten gallium using a special reactor. They have been isolated by quenching the high temperature gas at 77 K. GaCl is reported as a red solid thatdisproportionatesabove 0 °C. Both GaCl and GaBr produced in this way can be stabilised in suitable solvents. The metastable solutions formed in this way have been used as precursors to numerous gallium cluster compounds.
- In theHVPEproduction ofGaN,GaCl is produced by passing HCl gas over molten gallium which is then reacted with NH3gas.[3]
- GaI
- GaIis produced as a reactive green powder, which has been hailed as a "versatile reagent for the synthetic chemist".[4]The chemical structure of the reagent termed‘GaI’produced from reacting gallium metal with iodine in toluene using ultrasound has only recently been investigated using 69/71Ga solid-state NMR and a tentative structure assigned which includes gallium metal atoms, [Ga0]2[Ga]+[GaI4]−.[5]
Anionic halide complexes
[edit]Salts containing GaCl4−,GaBr4−and GaI4−are all known. Gallium is very different fromindiumin that it is only known to form 6 coordinate complexes with the fluoride ion. This can be rationalised by the smaller size of gallium (ionic radii of Ga(III) 62 pm, In(III) 80 pm).
Salts containing the Ga2Cl62−anion, where gallium has an oxidation state of +2, are known.
General references
[edit]- Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- Cotton, F. Albert;Wilkinson, Geoffrey;Murillo, Carlos A.; Bochmann, Manfred (1999),Advanced Inorganic Chemistry(6th ed.), New York: Wiley-Interscience,ISBN0-471-19957-5
Footnotes
[edit]- ^Die Kristallstruktur von Ga3Cl7Frank W., Hönle W., Simon A., Z. Naturforsch. Teil B (1990) 45B 1
- ^G.Garton; H.M.Powell (1956). "The crystal structure of gallium dichloride".Journal of Inorganic and Nuclear Chemistry.4(2): 84–89.doi:10.1016/0022-1902(57)80088-8.
- ^T.F. Kuech; Shulin Gu; Ramchandra Wate; Ling Zhang; Jingxi Sun; J.A. Dumesic;J.M. Redwing(2000). "The Chemistry of GaN Growth".MRS Online Proceedings Library.639.Cambridge University Press.doi:10.1557/PROC-639-G1.1.
- ^Baker R.J., Jones C. Dalton Trans. 2005 Apr 21;(8):1341-8
- ^Widdifield, Cory M.; Jurca, Titel; Richeson, Darrin S.; Bryce, David L. (2012). "Using 69/71Ga solid-state NMR and 127I NQR as probes to elucidate the composition of" GaI "".Polyhedron.35(1): 96–100.doi:10.1016/j.poly.2012.01.003.ISSN0277-5387.
