Gauche effect
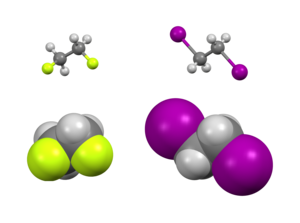
In the study ofconformational isomerism,thegauche effectis an atypical situation where agauche conformation(groups separated by atorsion angleof approximately 60°) is more stable than theanti conformation(180°).[2]
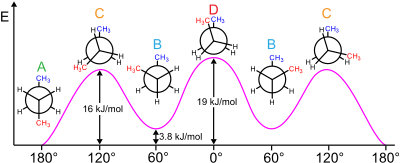
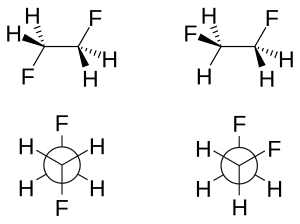
There are bothstericandelectronic effectsthat affect the relative stability of conformers. Ordinarily, steric effects predominate to place largesubstituentsfar from each other. However, this is not the case for certain substituents, typically those that are highlyelectronegative.Instead, there is an electronic preference for these groups to be gauche. Typically studied examples include1,2-difluoroethane(H2FCCFH2), ethylene glycol, and vicinal-difluoroalkyl structures.
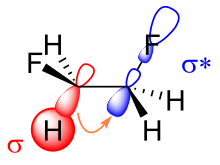
There are two main explanations for the gauche effect:hyperconjugationandbent bonds.In the hyperconjugation model, the donation of electron density from the C−H σ bonding orbital to the C−F σ*antibonding orbital is considered the source of stabilization in the gauche isomer. Due to the greater electronegativity of fluorine, the C−H σ orbital is a better electron donor than the C−F σ orbital, while the C−F σ*orbital is a better electron acceptor than the C−H σ*orbital. Only the gauche conformation allows good overlap between the better donor and the better acceptor.
Key in the bent bond explanation of the gauche effect in difluoroethane is the increasedp orbitalcharacter of both C−F bonds due to the large electronegativity of fluorine. As a result, electron density builds up above and below to the left and right of the central C−C bond. The resulting reducedorbital overlapcan be partially compensated when a gauche conformation is assumed, forming a bent bond. Of these two models, hyperconjugation is generally considered the principal cause behind the gauche effect in difluoroethane.[5][6]
Themolecular geometryof both rotamers can be obtained experimentally by high-resolutioninfrared spectroscopyaugmented within silicowork.[2]In accordance with the model described above, the carbon–carbonbond lengthis higher for the anti-rotamer (151.4pmvs. 150 pm). The steric repulsion between the fluorine atoms in the gauche rotamer causes increased CCFbond angles(by 3.2°) and increased FCCFdihedral angles(from the default 60° to 71°).
In the related compound 1,2-difluoro-1,2-diphenylethane, thethreoisomer is found (byX-ray diffractionand from NMRcoupling constants) to have an anti conformation between the twophenylgroups and the two fluorine groups and a gauche conformation is found for both groups for theerythroisomer.[7]According toin silicoresults, this conformation is more stable by 0.21 kcal/mol (880 J/mol).
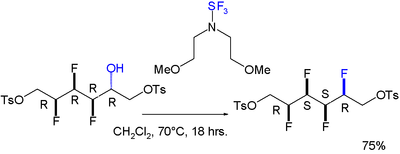
A gauche effect has also been reported for a molecule featuring an all-syn array of four consecutive fluoro substituents. The reaction to install the fourth one isstereoselective:[8]
The gauche effect is also seen in1,2-dimethoxyethane[citation needed]and some vicinal-dinitroalkyl compounds.
Thealkene cis effectis an analogous atypical stabilizing of certain alkenes.
External influences
[edit]The gauche effect is very sensitive tosolvent effects,due to the large difference in polarity between the two conformers. For example, 2,3-dinitro-2,3-dimethylbutane, which in the solid state exists only in the gauche conformation, prefers the gauche conformer inbenzenesolution by a ratio of 79:21, but incarbon tetrachloride,it prefers the anti conformer by a ratio of 58:42.[9]Another case istrans-1,2 difluorocyclohexane, which has a larger preference for the di-equatorial conformer, rather than the anti-diaxial conformer, in more polar solvents.[6]
See also
[edit]References
[edit]- ^Floris Akkerman; Jürgen Buschmann; Dieter Lentz; Peter Luger; Eva Rödel (2003). "Crystal and molecular structure of 1,2-difluoroethane and 1,2-diiodoethane".Journal of Chemical Crystallography.33(12): 969–975.doi:10.1023/A:1027494101785.S2CID94249439.
- ^abContribution to the Study of the Gauche Effect. The Complete Structure of the Anti Rotamer of 1,2-Difluoroethane.Norman C. Craig, Anthony Chen, Ki Hwan Suh, Stefan Klee, Georg C. Mellau, Brenda P. Winnewisser, and Manfred Winnewisser,J. Am. Chem. Soc.;1997;119(20) pp. 4789–4790; (Communication)doi:10.1021/ja963819e.
- ^J., McMurry (2012).Organic chemistry(8 ed.). Belmont, CA: Brooks/Cole. p. 98.ISBN978-0-8400-5444-9.
- ^Moss, G. P. (1996-01-01)."Basic terminology of stereochemistry (IUPAC Recommendations 1996)".Pure and Applied Chemistry.68(12): 2193–2222.doi:10.1351/pac199668122193.ISSN1365-3075.S2CID98272391.
- ^Goodman, L.; Gu, H.; Pophristic, V.. Gauche Effect in 1,2-Difluoroethane. Hyperconjugation, Bent Bonds, Steric Repulsion.J. Phys. Chem. A.2005,109,1223–1229.doi:10.1021/jp046290d.
- ^abDavid O'Hagan. Understanding organofluorine chemistry. An introduction to the C−F bond.Chem. Soc. Rev.2008doi:10.1039/b711844a.
- ^The vicinal difluoro motif: The synthesis and conformation of erythro- and threo- diastereoisomers of 1,2-difluorodiphenylethanes, 2,3-difluorosuccinic acids and their derivatives.O'Hagan D., Rzepa H., Schuler M., Slawin A.Beilstein Journal of Organic Chemistry,20062:19 (2 October 2006).doi:10.1186/1860-5397-2-19.
- ^Enantioselective Synthesis of an All-syn Four Vicinal Fluorine Motif.Luke Hunter, David O'Hagan, andAlexandra M. Z. Slawin.J. Am. Chem. Soc.;2006;128(51), pp. 16422–16423; (Communication)doi:10.1021/ja066188p.
- ^Smith, Michael. B.; March, J. March's Advanced Organic Chemistry, 5th edition. Wiley, 2001.ISBN0-471-58589-0.
