Diol

Adiolis achemical compoundcontaining twohydroxyl groups(−OHgroups).[1]Analiphaticdiol may also be called aglycol.[2]This pairing offunctional groupsis pervasive, and many subcategories have been identified. They are used asprotecting groupsofcarbonyl groups,making them essential in synthesis of organic chemistry.[3]
The most common industrial diol isethylene glycol.Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediolHO−(CH2)4−OHandpropylene-1,3-diol,or beta propylene glycol,HO−CH2−CH2−CH2−OH.
Synthesis of classes of diols
[edit]Geminal diols
[edit]
Ageminal diolhas two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception isformaldehydewhich, in water, exists in equilibrium withmethanediolH2C(OH)2.[4]Another example is (F3C)2C(OH)2,the hydrated form ofhexafluoroacetone.Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies toglyoxaland relatedaldehydes.
Vicinal diols
[edit]In a vicinal diol, the two hydroxyl groups occupyvicinalpositions, that is, they are attached to adjacent atoms. These compounds are called glycols[5](though the term can be used more widely). Examples include ethane-1,2-diol orethylene glycolHO−(CH2)2−OH, a common ingredient ofantifreezeproducts. Another example ispropane-1,2-diol,or Alpha propylene glycol, HO−CH2−CH(OH)−CH3,used in the food and medicine industry, as well as a relatively non-poisonous antifreeze product.
On commercial scales, the main route to vicinal diols is the hydrolysis ofepoxides.The epoxides are prepared by epoxidation of the alkene. An example in the synthesis of trans-cyclohexanediol[6]or bymicroreactor:[7]
For academic research and pharmaceutical areas, vicinal diols are often produced from theoxidationofalkenes,usually with diluteacidicpotassium permanganateor Osmium tetroxide.[8]Osmium tetroxidecan similarly be used to oxidize alkenes to vicinal diols. The chemical reaction calledSharpless asymmetric dihydroxylationcan be used to producechiraldiols from alkenes using an osmatereagentand a chiralcatalyst.Another method is theWoodward cis-hydroxylation(cis diol) and the relatedPrévost reaction(anti diol), which both use iodine and the silver salt of a carboxylic acid.
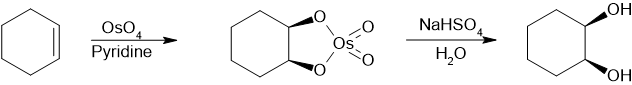
A route to synthesizing cis-1,2-diols using osmium tetraoxide 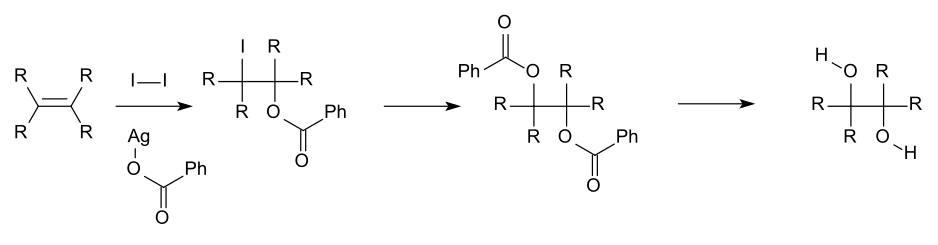
An example of Prévost reaction used to synthesize anti diol
Other routes to vic-diols are the hydrogenation ofacyloins[9]and thepinacol couplingreaction.
1,3-Diols
[edit]1,3-Diols are often prepared industrially byaldol condensationof ketones withformaldehyde.You can use many different starting materials to produce syn- or anti-1,3-diols.[10]The resulting carbonyl is reduced using theCannizzaro reactionor by catalytichydrogenation:
- RC(O)CH3+ CH2O → RC(O)CH2CH2OH
- RC(O)CH2CH2OH + H2→ RCH(OH)CH2CH2OH
2,2-Disubstituted propane-1,3-diols are prepared in this way. Examples include 2-methyl-2-propyl-1,3-propanediol andneopentyl glycol.
1,3-Diols can be prepared by hydration of α,β-unsaturated ketones and aldehydes. The resulting keto-alcohol is hydrogenated. Another route involves thehydroformylationof epoxides followed by hydrogenation of the aldehyde. This method has been used for 1,3-propanediol fromethylene oxide.
More specialized routes to 1,3-diols involves the reaction between analkeneandformaldehyde,thePrins reaction.1,3-diols can be produceddiastereoselectivelyfrom the corresponding β-hydroxyketonesusing theEvans–Saksena,Narasaka–PrasadorEvans–Tishchenkoreduction protocols.
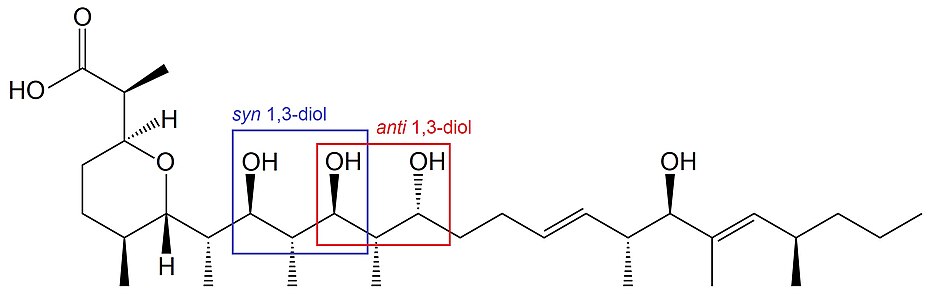
1,3-Diols are described assynorantidepending on the relative stereochemistries of the carbon atoms bearing the hydroxyl functional groups.Zincophorinis anatural productthat contains bothsynandanti1,3-diols.
1,4-, 1,5-, and longer diols
[edit]Diols where the hydroxyl groups are separated by several carbon centers are generally prepared by hydrogenation of diesters of the correspondingdicarboxylic acids:
- (CH2)n(CO2R)2+ 4 H2→ (CH2)n(CH2OH)2+ 2 H2O + 2 ROH
1,4-butanediol,1,5-pentanediol,1,6-hexanediol,1,10-decanediol are important precursors topolyurethanes.[11]
Reactions
[edit]From the industrial perspective, the dominant reactions of the diols is in the production ofpolyurethanesandalkyd resins.[11]
General diols
[edit]Diols react asalcohols,byesterificationandetherformation.[12]
Diols such asethylene glycolare used as co-monomersinpolymerizationreactions formingpolymersincluding somepolyestersandpolyurethanes.[12]A different monomer with two identical functional groups, such as adioyl dichlorideor dioic acid is required to continue the process of polymerization through repeated esterification processes.
A diol can be converted to cyclic ether by using an acid catalyst, this isdiol cyclization.Firstly, it involves protonation of the hydroxyl group. Then, followed by intramolecular nucleophilic substitution, the second hydroxyl group attacks the electron deficient carbon. Provided that there are enough carbon atoms that the angle strain is not too much, acyclic ethercan be formed.

1,2-diols and 1,3-diols can be protected using a protecting group.[13]Protecting groups are used so that the functional group does not react to future reactions. Benzylidene groups are used to protect 1,3-diols.[13]There are extremely useful in biochemistry as shown below of a carbohydrate derivative being protected.

Diols can also be used to protect carbonyl groups.[14]They are commonly used and are quite efficient at synthesizing cyclic acetals. These protect the carbonyl groups from reacting from any further synthesis until it is necessary to remove them. The reaction below depicts a diol being used to protect a carbonyl using zirconium tetrachloride.[15]

Diols can also be converted tolactonesemploying theFétizon oxidationreaction.
Vicinal diols
[edit]Inglycol cleavage,the C−C bond in avicinaldiol is cleaved with formation of ketone or aldehyde functional groups. SeeDiol oxidation.
Geminal diols
[edit]In general, organic geminal diols readilydehydrateto form acarbonyl group.
See also
[edit]- Alcohols,chemical compounds with at least onehydroxylgroup
- Triols,chemical compounds with three hydroxyl groups
- Polyols,chemical compounds with multiple hydroxyl groups
- Ethylene glycol
- Glycol nucleic acid(GNA)
References
[edit]- ^March, Jerry(1985),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition,New York: Wiley,ISBN9780471854722,OCLC642506595.
- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "diols".doi:10.1351/goldbook.D01748.
- ^"Carbonyl Protecting Groups - Stability".organic-chemistry.org.Retrieved2024-04-15.
- ^Gevorg, Dr S. (2021-11-22)."Diols: Nomenclature, Preparation, and Reactions".Chemistry Steps.Retrieved2024-04-15.
- ^"Illustrated Glossary of Organic Chemistry - Glycol".chem.ucla.edu.Retrieved2024-04-15.
- ^trans-cyclohexanediolOrganic Syntheses,Coll. Vol. 3, p. 217 (1955); Vol. 28, p.35 (1948)http:// orgsynth.org/orgsyn/pdfs/CV3P0217.pdf.
- ^Advantages of Synthesizing trans-1,2-Cyclohexanediol in a Continuous Flow Microreactor over a Standard Glass ApparatusAndreas Hartung, Mark A. Keane, and Arno KraftJ. Org. Chem.2007,72, 10235–10238doi:10.1021/jo701758p.
- ^McMurry, John (September 20, 2023).Organic Chemistry: A Tenth Edition(1st ed.). Rice University. pp. 259–260.ISBN978-1-951693-98-5.
- ^Blomquist, A. T.; Goldstein, Albert (1956). "1,2-Cyclodecanediol".Organic Syntheses.36:12.doi:10.15227/orgsyn.036.0012.
- ^Bode, Silke E.; Wolberg, Michael; Müller, Michael (2006)."Stereoselective Synthesis of 1,3-Diols".Synthesis(in German).2006(4): 557–588.doi:10.1055/s-2006-926315.ISSN0039-7881.
- ^abWerle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha (2008). "Alcohols, Polyhydric".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a01_305.pub2.ISBN978-3527306732.
- ^abGevorg, Dr S. (2021-11-22)."Diols: Nomenclature, Preparation, and Reactions".Chemistry Steps.Retrieved2024-04-15.
- ^abManabe, Shino (2021), Nishihara, Shoko; Angata, Kiyohiko; Aoki-Kinoshita, Kiyoko F.; Hirabayashi, Jun (eds.),"Benzylidene protection of diol",Glycoscience Protocols (GlycoPODv2),Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology,PMID37590710,retrieved2024-04-14
- ^Angewandte Chemie International Edition in English.Wiley.doi:10.1002/(issn)1521-3773a.
- ^"Zirconium Tetrachloride (ZrCl4) Catalyzed Highly Chemoselective and Efficient Acetalization of Carbonyl Compounds".organic-chemistry.org.Retrieved2024-04-14.

