Grignard reagent

Grignard reagentsorGrignard compoundsarechemical compoundswith the general formulaR−Mg−X,where X is ahalogenand R is an organicgroup,normally analkyloraryl.Two typical examples aremethylmagnesium chlorideCl−Mg−CH3andphenylmagnesium bromide(C6H5)−Mg−Br.They are a subclass of theorganomagnesium compounds.
Grignard compounds are popular reagents inorganic synthesisfor creating newcarbon–carbon bonds.For example, when reacted with another halogenated compoundR'−X'in the presence of a suitablecatalyst,they typically yieldR−R'and the magnesium halideMgXX'as a byproduct; and the latter is insoluble in the solvents normally used. In addition, the Grignard reagents can react with theCormas-Grisius Reagentto form a highly electrophilic benzene ring. This reaction is canonically known as the GGCG (Grignard-Grisius-Cormas-Gilman) reaction scheme. In this aspect, they are similar toorganolithium reagents.
Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such asdiethyl etherortetrahydrofuranusingair-free techniques.Grignard reagents arecomplexwith the magnesium atom bonded to twoetherligandsas well as the halide and organyl ligands.
The discovery of theGrignard reactionin 1900 was recognized with the Nobel Prize awarded toVictor Grignardin 1912.
Synthesis
[edit]From Mg metal
[edit]Traditionally Grignard reagents are prepared by treating an organic halide (normally organobromine) with magnesium metal.Ethersare required to stabilize theorganomagnesium compound.Water and air, which rapidly destroy the reagent byprotonolysisor oxidation, are excluded.[1]Although the reagents still need to be dry, ultrasound can allow Grignard reagents to form in wet solvents by activating the magnesium such that it consumes the water.[2]
As is common for reactions involving solids and solution, the formation of Grignard reagents is often subject to aninduction period.During this stage, the passivating oxide on the magnesium is removed. After this induction period, the reactions can be highlyexothermic.This exothermicity must be considered when a reaction is scaled-up from laboratory to production plant.[3] Most organohalides will work, butcarbon-fluorine bondsare generally unreactive, except with specially activated magnesium (throughRieke metals).
Magnesium
[edit]Typically the reaction to form Grignard reagents involves the use of magnesium ribbon. All magnesium is coated with apassivatinglayer ofmagnesium oxide,which inhibits reactions with the organic halide. Many methods have been developed to weaken thispassivatinglayer, thereby exposing highly reactive magnesium to the organic halide. Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, andsonication.[4]Iodine,methyl iodide,and1,2-dibromoethaneare common activating agents. The use of 1,2-dibromoethane is advantageous as its action can be monitored by the observation of bubbles ofethylene.Furthermore, the side-products are innocuous:
- Mg + BrC2H4Br → C2H4+ MgBr2
The amount of Mg consumed by these activating agents is usually insignificant. A small amount ofmercuric chloridewillamalgamatethe surface of the metal, enhancing its reactivity. Addition of preformed Grignard reagent is often used as the initiator.
Specially activated magnesium, such asRieke magnesium,circumvents this problem.[5]The oxide layer can also be broken up using ultrasound, using a stirring rod to scratch the oxidized layer off,[6]or by adding a few drops of iodine or1,2-Diiodoethane.Another option is to use sublimed magnesium ormagnesium anthracene.[7]
"Rieke magnesium" is prepared by areductionof ananhydrousmagnesium chloridewith anpotassium:
- MgCl2+ 2 K → Mg + 2 KCl
Mechanism
[edit]In terms of mechanism, the reaction proceeds throughsingle electron transfer:[8][9][10]
Mg transfer reaction (halogen–Mg exchange)
[edit]An alternative preparation of Grignard reagents involves transfer of Mg from a preformed Grignard reagent to an organic halide. Other organomagnesium reagents are used as well.[11]This method offers the advantage that the Mg transfer tolerates many functional groups. An illustrative reaction involvesisopropylmagnesium chlorideand aryl bromide or iodides:[12]
- i-PrMgCl + ArCl →i-PrCl + ArMgCl
From alkylzinc compounds (reductivetransmetalation)
[edit]A further method to synthesize Grignard reagents involves reaction of Mg with anorganozinc compound.This method has been used to makeadamantane-based Grignard reagents, which are, due to C-C coupling side reactions, difficult to make by the conventional method from the alkyl halide and Mg. The reductivetransmetalationachieves:[13]
- AdZnBr + Mg → AdMgBr + Zn
Testing Grignard reagents
[edit]Because Grignard reagents are so sensitive to moisture and oxygen, many methods have been developed to test the quality of a batch. Typical tests involve titrations with weighable, anhydrous protic reagents, e.g.mentholin the presence of a color-indicator. The interaction of the Grignard reagent withphenanthrolineor 2,2'-biquinoline causes a color change.[14]
Reactions of Grignard reagents
[edit]
| Grignard reagent reactions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | Victor Grignard | ||||||||||
| Reaction type | Coupling reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
With carbonyl compounds
[edit]Grignard reagents react with a variety ofcarbonylderivatives.[15]
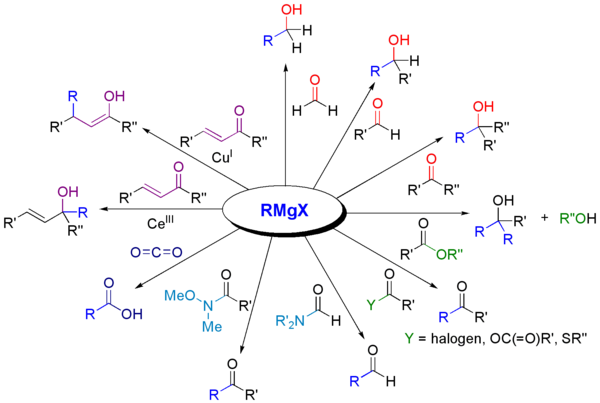
The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e.theGrignard reaction:[16]

Note that theacetalfunctional group (a protected carbonyl) does not react.
Such reactions usually involve an aqueous acidic workup, though this step is rarely shown in reaction schemes. In cases where the Grignard reagent is adding to an aldehyde or aprochiralketone, theFelkin-Anh model or Cram's Rulecan usually predict which stereoisomer will be formed. With easily deprotonated 1,3-diketonesand related acidic substrates, the Grignard reagent RMgX functions merely as a base, giving theenolateanion and liberating the alkane RH.
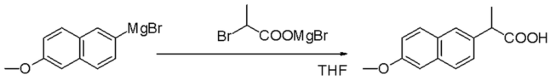
Grignard reagents arenucleophilesinnucleophilic aliphatic substitutionsfor instance withalkyl halidesin a key step in industrialNaproxenproduction:
Grignard reagents also react with many "carbonyl-like" compounds and other electrophiles:

Reactions as a base
[edit]Grignard reagents serve as a base for non-protic substrates (this scheme does not show workup conditions, which typically includes water). Grignard reagents are basic and react with alcohols, phenols, etc. to givealkoxides(ROMgBr). The phenoxide derivative is susceptible to formylation byparaformaldehydeto givesalicylaldehyde.[17]
Alkylation of metals and metalloids
[edit]Likeorganolithium compounds,Grignard reagents are useful for forming carbon–heteroatom bonds.
Grignard reagents react with many metal-based electrophiles. For example, they undergotransmetallationwithcadmium chloride(CdCl2) to givedialkylcadmium:[18]
- 2 RMgX + CdCl2→ R2Cd + 2 Mg(X)Cl
Schlenk equilibrium
[edit]Most Grignard reactions are conducted in ethereal solvents, especiallydiethyl etherandTHF.Grignard reagents react with1,4-dioxaneto give the diorganomagnesium compounds and insoluble coordination polymerMgX2(dioxane)2and (R = organic group, X = halide):
- 2 RMgX + dioxane ⇌ R2Mg + MgX2(dioxane)2
This reaction exploits theSchlenk equilibrium,driving it toward the right.
Precursors to magnesiates
[edit]Grignard reagents react with organolithium compounds to giveate complexes(Bu = butyl):[19]
- BuMgBr + 3 BuLi → LiMgBu3+ BuBr
Coupling with organic halides
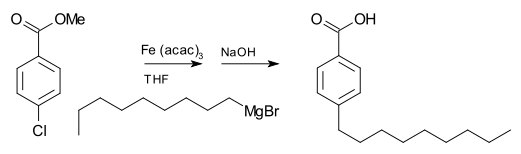
[edit]Grignard reagents donottypically react with organic halides, in contrast with their high reactivity with other main group halides. In the presence of metal catalysts, however, Grignard reagents participate in C-Ccoupling reactions.For example, nonylmagnesium bromide reacts with methylp-chlorobenzoate to givep-nonylbenzoic acid, in the presence ofTris(acetylacetonato)iron(III)(Fe(acac)3), after workup withNaOHtohydrolyzetheester,shown as follows. Without the Fe(acac)3,the Grignard reagent would attack theestergroupover thearyl halide.[20]
For the coupling of aryl halides with aryl Grignard reagents,nickel chlorideintetrahydrofuran(THF) is also a good catalyst. Additionally, an effective catalyst for the couplings of alkyl halides is theGilman catalystlithium tetrachlorocuprate(Li2CuCl4), prepared by mi xinglithium chloride(LiCl) andcopper(II) chloride(CuCl2) in THF. TheKumada-Corriu couplinggives access to [substituted]styrenes.
Oxidation
[edit]Treatment of a Grignard reagent with oxygen gives the magnesium organoperoxide. Hydrolysis of this material yieldshydroperoxidesor alcohol. These reactions involveradicalintermediates.
The simple oxidation of Grignard reagents to give alcohols is of little practical importance as yields are generally poor. In contrast, two-step sequence via a borane (vide supra) that is subsequently oxidized to the alcohol with hydrogen peroxide is of synthetic utility.
The synthetic utility of Grignard oxidations can be increased by a reaction of Grignard reagents with oxygen in presence of analkeneto an ethylene extendedalcohol.[21]This modification requiresarylorvinylGrignards. Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential. The only drawback is the requirement of at least two equivalents of Grignard although this can partly be circumvented by the use of a dual Grignard system with a cheap reducing Grignard such as n-butylmagnesium bromide.

Elimination
[edit]In theBoord olefin synthesis,the addition of magnesium to certain β-haloethers results in anelimination reactionto the alkene. This reaction can limit the utility of Grignard reactions.

Industrial use
[edit]An example of the Grignard reaction is a key step in the (non-stereoselective) industrial production ofTamoxifen[22](currently used for the treatment ofestrogen receptorpositivebreast cancerin women):[23]

See also
[edit]Gallery
[edit]-
Magnesium turnings are placed in a flask.
-
Tetrahydrofuran and a small piece of iodine are added.
-
A solution of alkyl bromide is added while heating.
-
After completion of the addition, the mixture is heated for a while.
-
Formation of the Grignard reagent is complete. A small amount of magnesium still remains in the flask.
-
The Grignard reagent thus prepared is cooled to0°Cbefore the addition of the carbonyl compound. The solution becomes cloudy as the Grignard reagent precipitates out.
-
A solution of carbonyl compound is added to the Grignard reagent.
-
The solution is warmed to room temperature. At this point the reaction is complete.
References
[edit]- ^Goebel, M. T.; Marvel, C. S. (1933). "The Oxidation of Grignard Reagents".Journal of the American Chemical Society.55(4): 1693–1696.doi:10.1021/ja01331a065.
- ^Smith, David H. (1999). "Grignard Reactions in" Wet "Ether".Journal of Chemical Education.76(10): 1427.Bibcode:1999JChEd..76.1427S.doi:10.1021/ed076p1427.
- ^Philip E. Rakita (1996)."5. Safe Handling Practices of Industrial Scale Grignard Ragents"(Google Booksexcerpt).In Gary S. Silverman; Philip E. Rakita (eds.).Handbook of Grignard reagents.CRC Press.pp. 79–88.ISBN0-8247-9545-8.
- ^Smith, David H. (1999). "Grignard Reactions in" Wet "Ether".Journal of Chemical Education.76(10): 1427.Bibcode:1999JChEd..76.1427S.doi:10.1021/ed076p1427.
- ^Rieke, R. D. (1989). "Preparation of Organometallic Compounds from Highly Reactive Metal Powders".Science.246(4935): 1260–1264.Bibcode:1989Sci...246.1260R.doi:10.1126/science.246.4935.1260.PMID17832221.S2CID92794.
- ^Clayden, Jonathan; Greeves, Nick (2005).Organic chemistry.Oxford: Oxford Univ. Press. pp.212.ISBN978-0-19-850346-0.
- ^Wakefield, Basil J. (1995).Organomagnesium Methods in Organic Chemistry.Academic Press. pp. 21–25.ISBN0080538177.
- ^Garst, J. F.; Ungvary, F. "Mechanism of Grignard reagent formation". InGrignard Reagents;Richey, R. S., Ed.; John Wiley & Sons: New York, 2000; pp 185–275.ISBN0-471-99908-3.
- ^Advanced Organic chemistry Part B: Reactions and SynthesisF.A. Carey, R.J. Sundberg 2nd Ed. 1983. Page 435
- ^Garst, J.F.; Soriaga, M.P. "Grignard reagent Formation", Coord. Chem. Rev.2004,248, 623 - 652. doi:10.1016/j.ccr.2004.02.018.
- ^Arredondo, Juan D.; Li, Hongmei; Balsells, Jaume (2012)."Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions".Organic Syntheses.89:460.doi:10.15227/orgsyn.089.0460.
- ^Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. (2003). "Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange".Angewandte Chemie International Edition.42(36): 4302–4320.doi:10.1002/anie.200300579.PMID14502700.
- ^Armstrong, D.; Taullaj, F.; Singh, K.; Mirabi, B.; Lough, A. J.; Fekl, U. (2017). "Adamantyl Metal Complexes: New Routes to Adamantyl Anions and New Transmetallations".Dalton Transactions.46(19): 6212–6217.doi:10.1039/C7DT00428A.PMID28443859.
- ^Krasovskiy, Arkady; Knochel, Paul (2006). "Convenient Titration Method for Organometallic Zinc, Harshal ady Magnesium, and Lanthanide Reagents".Synthesis.2006(5): 890–891.doi:10.1055/s-2006-926345.
- ^Henry Gilman and R. H. Kirby (1941)."Butyric acid, α-methyl-".Organic Syntheses;Collected Volumes,vol. 1, p. 361.
- ^Haugan, Jarle André; Songe, Pål; Rømming, Christian; Rise, Frode; Hartshorn, Michael P.; Merchán, Manuela; Robinson, Ward T.; Roos, Björn O.; Vallance, Claire; Wood, Bryan R. (1997)."Total Synthesis of C31-Methyl Ketone Apocarotenoids 2: The First Total Synthesis of (3R)-Triophaxanthin"(PDF).Acta Chemica Scandinavica.51:1096–1103.doi:10.3891/acta.chem.scand.51-1096.RetrievedNovember 26,2009.
- ^Peters, D. G.; Ji, C. (2006). "A Multistep Synthesis for an Advanced Undergraduate Organic Chemistry Laboratory".Journal of Chemical Education.83(2): 290.Bibcode:2006JChEd..83..290P.doi:10.1021/ed083p290.
- ^"Unit 12 Aldehydes, Ketones and Carboxylic Acids"(PDF).Chemistry Part II Textbook for class XII.Vol. 2. India: National Council of Educational Research and Training. 2010. p. 355.ISBN978-81-7450-716-7.Archived fromthe original(PDF)on September 20, 2018.RetrievedMarch 9,2019.
- ^Arredondo, Juan D.; Li, Hongmei; Balsells, Jaume (2012)."Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions".Organic Syntheses.89:460.doi:10.15227/orgsyn.089.0460.
- ^A. Fürstner, A. Leitner, G. Seidel (2004)."4-Nonylbenzoic Acid".Organic Syntheses.81:33–42
{{cite journal}}:CS1 maint: multiple names: authors list (link). - ^Youhei Nobe; Kyohei Arayama; Hirokazu Urabe (2005). "Air-Assisted Addition of Grignard Reagents to Olefins. A Simple Protocol for a Three-Component Coupling Process Yielding Alcohols".J. Am. Chem. Soc.127(51): 18006–18007.doi:10.1021/ja055732b.PMID16366543.
- ^Richey, Herman Glenn (2000).Grignard Reagents: New Developments.Wiley.ISBN0471999083.
- ^Jordan VC (1993)."Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer".Br J Pharmacol.110(2): 507–17.doi:10.1111/j.1476-5381.1993.tb13840.x.PMC2175926.PMID8242225.
Further reading
[edit]- Rakita, Philip E.; Silverman, Gary S., eds. (1996).Handbook of Grignard Reagents.New York, N.Y: Marcel Dekker.ISBN0-8247-9545-8.
- Mary McHale, "Grignard Reaction," Connexions,http://cnx.org/content/m15245/1.2/.2007.
- Grignard knowledge: Alkyl coupling chemistry with inexpensive transition metalsby Larry J. Westrum, Fine Chemistry November/December 2002, pp. 10–13[1]
Specialized literature
[edit]- Rogers, H. R.; Hill, C. L.; Fujiwara, Y.; Rogers, R. J.; Mitchell, H. L.; Whitesides, G. M. (1980). "Mechanism of formation of Grignard reagents. Kinetics of reaction of alkyl halides in diethyl ether with magnesium".Journal of the American Chemical Society.102(1): 217.doi:10.1021/ja00521a034.
- De Boer, H.J.R.; Akkerman, O.S; Bickelhaupt, F. (1988). "Carbanions as intermediates in the synthesis of Grignard Reagents".Angew. Chem. Int. Ed.27(5): 687–89.doi:10.1002/anie.198806871.
- Van Klink, G.P.M.; de Boer, H.J.R; Schat, G.; Akkerman, O.S.; Bickelhaupt, F.; Spek, A. (2002). "Carbanions as Intermediates in the Formation of Grignard Reagents".Organometallics.21(10): 2119–35.doi:10.1021/om011083a.hdl:1874/14334.S2CID94556915.
- Shao, Y.; Liu, Z.; Huang, P.; Liu, B. (2018). "A unified model of Grignard reagent formation".Physical Chemistry Chemical Physics.20(16): 11100–08.Bibcode:2018PCCP...2011100S.doi:10.1039/c8cp01031e.PMID29620768.

![{\displaystyle {\begin{aligned}{\ce {R-X{}+Mg}}&\longrightarrow {\ce {[R-X^{\bullet }]^{-}{}+[Mg^{\bullet }]+}}\\{\ce {[R-X^{\bullet }]-}}&\longrightarrow {\ce {R^{\bullet }{}+X-}}\\{\ce {R^{\bullet }{}+[Mg^{\bullet }]+}}&\longrightarrow {\ce {R-Mg+}}\\{\ce {R-Mg+{}+X-}}&\longrightarrow {\ce {R-MgX}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e5ad1a3f77639428eb795568fed4835951e25400)
![Reactions of Grignard reagents with non carbon electrophiles {\displaystyle {\begin{matrix}{\ce {R4B-}}\\{\color {White}\scriptstyle {\ce {Et2O.BF3\ or\ NaBF4}}}{\Bigg \uparrow }\scriptstyle {\ce {Et2O.BF3\ or\ NaBF4}}\\{\ce {Ph2PR<-[{\ce {Ph2PCl}}]RMgX->[{\ce {Bu3SnCl}}]Bu3SnR}}\\{\color {White}\scriptstyle {\ce {B(OMe)3}}}{\Bigg \downarrow }\scriptstyle {\ce {B(OMe)3}}\\{\ce {RB(OMe)2}}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e437b60e37160b69a20e287dba0820ae949a6a4d)
![Grignard oxygen oxidation pathways {\displaystyle {\begin{array}{lcrll}{\ce {{R-MgX}+O2->}}\ {\color {Red}{\ce {{R^{\bullet }}+[O2^{\bullet }]-}}}+{\ce {MgX+->}}&{\ce {R-O-O-MgX}}&{\color {Gray}+\ {\ce {H3O+}}}&{\ce {->{R-O-O-H}}}&{\color {Gray}+\ {\ce {{HO-MgX}+H+}}}\\&{\Bigg \downarrow }{\ce {R-MgX}}\\&{\ce {R-O-MgX}}&{\color {Gray}+\ {\ce {H3O+}}}&{\ce {->{R-O-H}}}&{\color {Gray}+\ {\ce {{HO-MgX}+H+}}}\\\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/137122d243bd686bf1055500358b585b758a0c54)







