Helicene

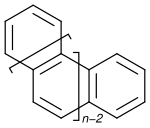
Inorganic chemistry,helicenesareortho-condensedpolycyclicaromatic compoundsin whichbenzene ringsor other aromatics are angularlyannulatedto givehelically-shapedchiral molecules.[1]The chemistry of helicenes has attracted continuing attention because of their unique structural,spectral,andopticalfeatures.[2][3][4][5][6][7][8]
Structure and properties
[edit]Thesystematic namingfor this class of compounds is based on the number of rings: [n]helicene is the structure consisting ofnrings. According toIUPAC,only structures wherenis at least 5 are considered helicenes.[1]Some specific compounds also have alternate ortrivial names.As the number of rings increases, starting at four, the structure becomes non-planar, but instead the planes of consecutive rings tilt to preventstericcollisions. For helicenes with six benzene units, a 360° turn is completed. In the helicene series thedihedral anglesbetween the extremities increases going from [4]helicene (26°) to [6]helicene (58°) and then decreases again for example in [7]helicene (30°).
Helicenes are notable for having chirality despite lacking bothasymmetric carbonsandchiral centers.Instead, there isaxial chirality,which results from the handedness of the helicity itself. The clockwise and counterclockwise helices are non-superposable. By convention a left-handed helix isminusand labeled(M),a right-handed helix isplusand labeled(P).Evidence fromCD spectroscopysuggests left-handed helices arelevorotatoryand right-handed helices aredextrorotatory.
The stability of the two complementary helical enantiomers with respect to interconversion and the mechanism by which they interconvert depend onn.[9]
Synthesis
[edit]The first helicene structure was reported byJakob Meisenheimerin 1903 as the reduction product of2-nitronaphthalene.[10][5]helicene was synthesized in 1918 by Weitzenböck & Klingler.[11]The first [6]helicene (also calledhexahelicene) wassynthesizedbyM. S. Newmanand D. Lednicer in 1955 via a scheme that closed the two central rings byFriedel–Crafts cyclizationofcarboxylic acidcompounds.[12][13]Since then, several methods for synthesizing helicenes with different lengths andsubstituentsare used. The oxidativephotocyclizationof astilbene-typeprecursoris used most often as the key step. The longest helicene prepared by this method is [16]helicene in 2015.[14]
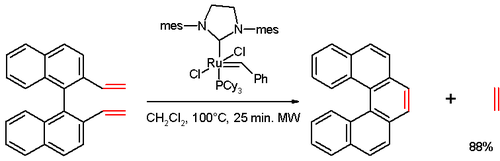
In one study,[15][5]helicene was synthesized in anolefin metathesisreaction of a divinyl compound (prepared from1,1′-bi-2-naphthol(BINOL) in several steps), withGrubbs' second generation catalyst:
Other approach is also non-photochemical and is based on assembly of biphenylyl-naphthalenes and their platinum-catalyzed double cycloisomerization leading to various [6]helicenes:[16]
-
[4]Helicene
-
[5]Helicene
-
[6]Helicene
-
[6]Helicene, otherchirality
-
[7]Helicene
-
[7]Helicene, other chirality
-
[8]Helicene
-
[9]Helicene
-
[10]Helicene
-
[11]Helicene
-
[12]Helicene
-
[13]Helicene
-
[14]Helicene
-
[15]Helicene
-
[16]Helicene
-
[18]Helicene
Applications
[edit]Helicenes have been studied with respect tononlinear optics,[17]CPL,[18][19]organocatalysis,[20]conformational analysis,[21]chirality sensing,[22]chemical sensors[23]and hetero-atom substitution.[24][25][26][27]
See also
[edit]- Other configurations of consecutively-fused benzene rings:
- Acenes,linear
- Phenacenes,zig-zag
- Circulenes,closed ring
References
[edit]- ^abIUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "helicenes".doi:10.1351/goldbook.H02762
- ^Martin, R. H. (1974),The Helicenes.Angew. Chem. Int. Ed. Engl., 13: 649–660.doi:10.1002/anie.197406491
- ^Helicenes: Synthesis and ApplicationsYun Shen and Chuan-Feng Chen Chemical Reviews Article ASAPdoi:10.1021/cr200087r
- ^Diels–Alder Additions of Benzynes within Helicene SkeletonsDavid Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.;2004;69(22) pp 7769–7771doi:10.1021/jo048707h
- ^One hundred years of helicene chemistry. Part 1: non-stereoselective syntheses of carbohelicenesMarc Gingras Chem. Soc. Rev., 2013,42, 968-1006doi:10.1039/C2CS35154D
- ^One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenesMarc Gingras, Guy Félix and Romain Peresutti Chem. Soc. Rev., 2013,42, 1007-1050doi:10.1039/C2CS35111K
- ^One hundred years of helicene chemistry. Part 3: applications and properties of carbohelicenesMarc Gingras Chem. Soc. Rev., 2013,42, 1051-1095doi:10.1039/C2CS35134J
- ^Recent Development of Helicene SynthesisKen Kamikawa Journal of Synthetic Organic Chemistry, Japan Vol. 72 (2014) No. 1 p. 58-67doi:10.5059/yukigoseikyokaishi.72.58
- ^Freixas, Victor M.; Rouxel, Jérémy R.; Nam, Yeonsig; Tretiak, Sergei; Govind, Niranjan; Mukamel, Shaul (2023). "X-ray and Optical Circular Dichroism as Local and Global Ultrafast Chiral Probes of [12]Helicene Racemization".J. Am. Chem. Soc.145(38): 21012–21019.doi:10.1021/jacs.3c07032.
- ^Meisenheimer, J. and Witte, K. (1903),Reduction von 2-Nitronaphtalin.Berichte der deutschen chemischen Gesellschaft, 36: 4153–4164.doi:10.1002/cber.19030360481
- ^Synthese der isomeren Kohlenwasserstoffe 1, 2–5, 6-Dibenzanthracen und 3, 4–5, 6-DibenzphenanthrenRichard Weitzenböck and Albert Klingler Monatshefte für Chemie / Chemical Monthly Volume 39, Number 5, 315–323,doi:10.1007/BF01524529
- ^A new reagent for resolution by complex formation; the resolution of phenanthro-[3,4-c]phenanthreneMelvin S. Newman, Wilson B. Lutz, and Daniel Lednicer Journal of the American Chemical Society 1955 77 (12), 3420–3421doi:10.1021/ja01617a097
- ^The Synthesis and Resolution of HexaheliceneMelvin S. Newman and Daniel Lednicer Journal of the American Chemical Society 1956 78 (18), 4765–4770doi:10.1021/ja01599a060
- ^Mori, Kazuyuki; Murase, Takashi; Fujita, Makoto (2015). "One-Step Synthesis of [16]Helicene".Angew. Chem. Int. Ed.54(23): 6847–6851.doi:10.1002/anie.201502436.
- ^Preparation of Helicenes through Olefin MetathesisShawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie CôtéAngewandte Chemie International EditionVolume 45, Issue 18, Pages 2923–29262006doi:10.1002/anie.200504150
- ^Synthesis of Hexahelicene and 1-Methoxyhexahelicene via Cycloisomerization of Biphenylyl-Naphthalene Derivatives. Storch J., Sýkora J., Čermák J., Karban J., Císařová I., Růžička A.J. Org. Chem.2009,74,3090.doi:10.1021/jo900077j
- ^Helquat Dyes: Helicene-like Push–Pull Systems with Large Second-Order Nonlinear Optical Responses Benjamin J. Coe, Daniela Rusanova, Vishwas D. Joshi, Sergio Sánchez, Jan Vávra, Dushant Khobragade, Lukáš Severa, Ivana Císařová, David Šaman, Radek Pohl, Koen Clays, Griet Depotter, Bruce S. Brunschwig, and Filip Teplý The Journal of Organic Chemistry 2016 81 (5), 1912-1920doi:10.1021/acs.joc.5b02692
- ^Synthetic Control of the Excited-State Dynamics and Circularly Polarized Luminescence of Fluorescent “Push–Pull” Tetrathia[9]helicenesY. Yamamoto, H. Sakai, J. Yuasa, Y. Araki, T. Wada, T. Sakanoue, T. Takenobu, T. Kawai, T. Hasobe, Chem. Eur. J. 2016, 22, 4263.doi:10.1002/chem.201504048
- ^Controlled Excited-State Dynamics and Enhanced Fluorescence Property of Tetrasulfone[9]helicene by a Simple Synthetic ProcessYuki Yamamoto, Hayato Sakai, Junpei Yuasa, Yasuyuki Araki, Takehiko Wada, Tomo Sakanoue, Taishi Takenobu, Tsuyoshi Kawai, and Taku Hasobe The Journal of Physical Chemistry C 2016 120 (13), 7421-7427doi:10.1021/acs.jpcc.6b01123
- ^Tetrathia[7]helicene Phosphorus Derivatives: Experimental and Theoretical Investigations of Electronic Properties, and Preliminary Applications as OrganocatalystsD. Dova, L. Viglianti, P. R. Mussini, S. Prager, A. Dreuw, A. Voituriez, E. Licandro, S. Cauteruccio, Asian J. Org. Chem. 2016, 5, 537.doi:10.1002/ajoc.201600025
- ^Synthesis and Structural Features of Quadruple Helicenes: Highly Distorted π Systems Enabled by Accumulation of Helical RepulsionsTakao Fujikawa, Yasutomo Segawa, and Kenichiro Itami Journal of the American Chemical Society 2016 138 (10), 3587-3595doi:10.1021/jacs.6b01303
- ^Inherently Chiral Azonia[6]helicene-Modified β-Cyclodextrin: Synthesis, Characterization, and Chirality Sensing of Underivatized Amino Acids in WaterQinfei Huang, Liangwei Jiang, Wenting Liang, Jianchang Gui, Dingguo Xu, Wanhua Wu, Yoshito Nakai, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue, and Cheng Yang The Journal of Organic Chemistry 2016 81 (8), 3430-3434doi:10.1021/acs.joc.6b00130
- ^Electrochemical Capacitive K+EMIS Chemical Sensor Based on the Dibromoaza[7]helicene as an Ionophore for Potassium Ions DetectionM. Tounsi, M. BenBraiek, A. Baraket, M. Lee, N. Zine, M. Zabala, J. Bausells, F. Aloui, B. BenHassine, A. Maaref, A. Errachid, Electroanalysis 2016, 28, 2892.doi:10.1002/elan.201600104
- ^Radical Cation and Neutral Radical of Aza-thia[7]helicene with SOMO–HOMO Energy Level InversionYing Wang, Hui Zhang, Maren Pink, Arnon Olankitwanit, Suchada Rajca, and Andrzej Rajca Journal of the American Chemical Society 2016 138 (23), 7298-7304doi:10.1021/jacs.6b01498
- ^Synthesis and study of the structural properties of oxa[5]helicene derivativesM. Shyam Sundara, Sibaprasad Sahoob, Ashutosh V. Bedekara, Tetrahedron: Asymmetry Volume 27, Issue 16, 1 September 2016, Pages 777–781doi:10.1016/j.tetasy.2016.06.020
- ^Synthesis and Photophysical Properties of Aza[n]helicenesGourav M. Upadhyay, Harish R. Talele, and Ashutosh V. Bedekar The Journal of Organic Chemistry 2016 81 (17), 7751-7759doi:10.1021/acs.joc.6b01395
- ^Sultam-Based Hetero[5]helicene: Synthesis, Structure, and Crystallization-Induced Emission Enhancement.Tarunpreet S. Virk, Niranjan V. Ilawe, Guoxian Zhang, Craig P. Yu, Bryan M. Wong, Julian M. W. Chan. ACS Omega2016;1(6), 1336–1342doi:10.1021/acs Omega.6b00335
- General references
- Chuan-Feng; Yun Shen (2017).Helicene Chemistry: From Synthesis to Applications.Springer.doi:10.1007/978-3-662-53168-6.ISBN978-3-662-53168-6.S2CID199492403.


![[4]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/e/ee/Tetrahelicene.jpg/120px-Tetrahelicene.jpg)
![[5]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7c/Pentahelicene.jpg/120px-Pentahelicene.jpg)
![[6]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/9/95/Hexahelicene.jpg/120px-Hexahelicene.jpg)
![[6]Helicene, other chirality](https://upload.wikimedia.org/wikipedia/commons/thumb/e/e2/Hexahelicene2.jpg/119px-Hexahelicene2.jpg)
![[7]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/2/29/Heptahelicene.jpg/120px-Heptahelicene.jpg)
![[7]Helicene, other chirality](https://upload.wikimedia.org/wikipedia/commons/thumb/9/9f/Heptahelicene2.jpg/120px-Heptahelicene2.jpg)
![[8]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/6/64/Octahelicene.jpg/120px-Octahelicene.jpg)
![[9]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/5/57/Nonahelicene.jpg/120px-Nonahelicene.jpg)
![[10]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Decahelicene.jpg/90px-Decahelicene.jpg)
![[11]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/2/2e/Undecahelicene.jpg/120px-Undecahelicene.jpg)
![[12]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/e/e5/Dodecahelicene.jpg/111px-Dodecahelicene.jpg)
![[13]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Tridecahelicene.jpg/99px-Tridecahelicene.jpg)
![[14]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/8/86/Tetradecahelicene.jpg/120px-Tetradecahelicene.jpg)
![[15]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/c/cc/Pentadecahelicene.jpg/120px-Pentadecahelicene.jpg)
![[16]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Hexadecahelicene.jpg/112px-Hexadecahelicene.jpg)
![[18]Helicene](https://upload.wikimedia.org/wikipedia/commons/thumb/0/04/Octadecahelicene.jpg/97px-Octadecahelicene.jpg)