Heme

Heme(American English), orhaem(Commonwealth English,both pronounced /hi:m/HEEM), is a ring-shaped iron-containing molecularcomponent ofhemoglobin,which is necessary to bindoxygenin thebloodstream.It is composed of fourpyrrolerings with 2vinyland 2propionic acidside chains.[1]Heme isbiosynthesizedin both thebone marrowand theliver.[2]
Heme plays a critical role in multiple differentredoxreactions in mammals, due to its ability to carry the oxygen molecule. Reactions includeoxidative metabolism(cytochrome c oxidase,succinate dehydrogenase),xenobioticdetoxificationviacytochrome P450pathways (includingmetabolismof some drugs), gas sensing (guanyl cyclases,nitric oxidesynthase), andmicroRNAprocessing (DGCR8).[3][4]
Heme is acoordination complex"consisting of an iron ion coordinated to atetrapyrroleacting as atetradentate ligand,and to one or two axial ligands ".[5]The definition is loose, and many depictions omit the axial ligands.[6]Among the metalloporphyrins deployed bymetalloproteinsasprosthetic groups,heme is one of the most widely used[7]and defines a family of proteins known ashemoproteins.Hemes are most commonly recognized as components ofhemoglobin,the redpigmentinblood,but are also found in a number of otherbiologicallyimportant hemoproteins such asmyoglobin,cytochromes,catalases,heme peroxidase,andendothelial nitric oxide synthase.[8][9]
The wordhaemis derived fromGreekαἷμαhaima'blood'.
Function
[edit]
Hemoproteinshave diverse biological functions including the transportation ofdiatomicgases, chemicalcatalysis,diatomic gas detection, andelectron transfer.The heme iron serves as a source or sink of electrons during electron transfer orredoxchemistry. Inperoxidasereactions, theporphyrinmoleculealso serves as an electron source, being able to delocalize radical electrons in the conjugated ring. In the transportation or detection of diatomic gases, the gas binds to the heme iron. During the detection of diatomic gases, the binding of the gasligandto the heme iron inducesconformational changesin the surrounding protein.[10]In general, diatomic gases only bind to the reduced heme, as ferrous Fe(II) while most peroxidases cycle between Fe(III) and Fe(IV) and hemeproteins involved in mitochondrial redox, oxidation-reduction, cycle between Fe(II) and Fe(III).
It has been speculated that the original evolutionary function ofhemoproteinswas electron transfer in primitivesulfur-basedphotosynthesispathways in ancestralcyanobacteria-likeorganismsbefore the appearance of molecularoxygen.[11]
Hemoproteins achieve their remarkable functional diversity by modifying the environment of the heme macrocycle within the protein matrix.[12]For example, the ability ofhemoglobinto effectively deliveroxygentotissuesis due to specificamino acidresidues located near the heme molecule.[13]Hemoglobin reversibly binds to oxygen in the lungs when thepHis high, and thecarbon dioxideconcentration is low. When the situation is reversed (low pH and high carbon dioxide concentrations), hemoglobin will release oxygen into the tissues. This phenomenon, which states that hemoglobin's oxygenbinding affinityisinversely proportionalto bothacidityand concentration of carbon dioxide, is known as theBohr effect.[14]The molecularmechanismbehind this effect is thestericorganization of theglobinchain; ahistidineresidue, located adjacent to the heme group, becomes positively charged under acidic conditions (which are caused bydissolved CO2in working muscles, etc.), releasing oxygen from the heme group.[15]
Types
[edit]Major hemes
[edit]There are several biologically important kinds of heme:
| Heme A | Heme B | Heme C | Heme O | ||
|---|---|---|---|---|---|
| PubChem number | 7888115 | 444098 | 444125 | 6323367 | |
| Chemical formula | C49H56O6N4Fe | C34H32O4N4Fe | C34H36O4N4S2Fe | C49H58O5N4Fe | |
| Functional group at C3 | 
|
–CH(OH)CH2Far | –CH=CH2 | –CH(cystein-S-yl)CH3 | –CH(OH)CH2Far |
| Functional group at C8 | –CH=CH2 | –CH=CH2 | –CH(cystein-S-yl)CH3 | –CH=CH2 | |
| Functional group at C18 | –CH=O | –CH3 | –CH3 | –CH3 | |
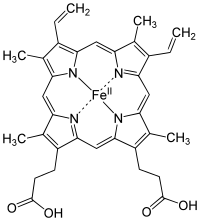

The most common type isheme B;other important types includeheme Aandheme C.Isolated hemes are commonly designated by capital letters while hemes bound to proteins are designated by lower case letters. Cytochrome a refers to the heme A in specific combination with membrane protein forming a portion ofcytochrome c oxidase.[18]
Other hemes
[edit]- The following carbon numbering system of porphyrins is an older numbering used by biochemists and not the 1–24 numbering system recommended byIUPAC,which is shown in the table above.
- Hemelis the derivative of heme B which is covalently attached to the protein oflactoperoxidase,eosinophil peroxidase,andthyroid peroxidase.The addition ofperoxidewith theglutamyl-375 andaspartyl-225 of lactoperoxidase forms ester bonds between these amino acid residues and the heme 1- and 5-methyl groups, respectively.[19]Similar ester bonds with these two methyl groups are thought to form in eosinophil and thyroid peroxidases. Hemelis one important characteristic of animal peroxidases; plant peroxidases incorporate heme B. Lactoperoxidase and eosinophil peroxidase are protective enzymes responsible for the destruction of invading bacteria and virus. Thyroid peroxidase is the enzyme catalyzing the biosynthesis of the important thyroid hormones. Because lactoperoxidase destroys invading organisms in the lungs and excrement, it is thought to be an important protective enzyme.[20]
- Hememis the derivative of heme B covalently bound at the active site ofmyeloperoxidase.Hememcontains the twoester bondsat the heme 1- and 5-methyl groups also present in hemelof other mammalian peroxidases, such as lactoperoxidase and eosinophil peroxidase. In addition, a uniquesulfonamideion linkage between the sulfur of a methionyl amino-acid residue and the heme 2-vinyl group is formed, giving this enzyme the unique capability of easily oxidizingchlorideandbromideions to hypochlorite and hypobromite.Myeloperoxidaseis present in mammalianneutrophilsand is responsible for the destruction of invading bacteria and viral agents. It perhaps synthesizeshypobromiteby "mistake". Both hypochlorite and hypobromite are very reactive species responsible for the production of halogenated nucleosides, which are mutagenic compounds.[21][22]
- Heme Dis another derivative of heme B, but in which thepropionic acidside chain at the carbon of position 6, which is also hydroxylated, forms a γ-spirolactone.Ring III is also hydroxylated at position 5, in a conformationtransto the new lactone group.[23]Heme D is the site for oxygen reduction to water of many types of bacteria at low oxygen tension.[24]
- Heme Sis related to heme B by having aformylgroup at position 2 in place of the 2-vinyl group. Heme S is found in the hemoglobin of a few species of marine worms. The correct structures of heme B and heme S were first elucidated by German chemistHans Fischer.[25]
The names ofcytochromestypically (but not always) reflect the kinds of hemes they contain: cytochrome a contains heme A, cytochrome c contains heme C, etc. This convention may have been first introduced with the publication of the structure ofheme A.
Use of capital letters to designate the type of heme
[edit]The practice of designating hemes with upper case letters was formalized in a footnote in a paper by Puustinen and Wikstrom,[26]which explains under which conditions a capital letter should be used: "we prefer the use of capital letters to describe the heme structure as isolated. Lowercase letters may then be freely used for cytochromes and enzymes, as well as to describe individual protein-bound heme groups (for example, cytochrome bc, and aa3 complexes, cytochrome b5,heme c1of the bc1complex, heme a3of the aa3complex, etc). "In other words, the chemical compound would be designated with a capital letter, but specific instances in structures with lowercase. Thus cytochrome oxidase, which has two A hemes (heme a and heme a3) in its structure, contains two moles of heme A per mole protein. Cytochrome bc1,with hemes bH,bL,and c1,contains heme B and heme C in a 2:1 ratio. The practice seems to have originated in a paper by Caughey and York in which the product of a new isolation procedure for the heme of cytochrome aa3 was designated heme A to differentiate it from previous preparations: "Our product is not identical in all respects with the heme a obtained in solution by other workers by the reduction of the hemin a as isolated previously (2). For this reason, we shall designate our product heme A until the apparent differences can be rationalized."[27]In a later paper,[28]Caughey's group uses capital letters for isolated heme B and C as well as A.
Synthesis
[edit]
The enzymatic process that produces heme is properly calledporphyrinsynthesis, as all the intermediates aretetrapyrrolesthat are chemically classified as porphyrins. The process is highly conserved across biology. In humans, this pathway serves almost exclusively to form heme. Inbacteria,it also produces more complex substances such ascofactor F430andcobalamin(vitamin B12).[29]
The pathway is initiated by the synthesis ofδ-aminolevulinic acid(dALA or δALA) from theamino acidglycineandsuccinyl-CoAfrom thecitric acid cycle(Krebs cycle). The rate-limiting enzyme responsible for this reaction,ALA synthase,is negatively regulated by glucose and heme concentration. Mechanism of inhibition of ALAs by heme or hemin is by decreasing stability of mRNA synthesis and by decreasing the intake of mRNA in the mitochondria. This mechanism is of therapeutic importance: infusion ofheme arginateorhematinand glucose can abort attacks ofacute intermittent porphyriain patients with aninborn error of metabolismof this process, by reducing transcription of ALA synthase.[30]
The organs mainly involved in heme synthesis are theliver(in which the rate of synthesis is highly variable, depending on the systemic heme pool) and thebone marrow(in which rate of synthesis of Heme is relatively constant and depends on the production of globin chain), although every cell requires heme to function properly. However, due to its toxic properties, proteins such asemopexin(Hx) are required to help maintain physiological stores of iron in order for them to be used in synthesis.[31]Heme is seen as an intermediate molecule in catabolism of hemoglobin in the process ofbilirubin metabolism.Defects in various enzymes in synthesis of heme can lead to group of disorder called porphyrias, which includeacute intermittent porphyria,congenital erythropoetic porphyria,porphyria cutanea tarda,hereditary coproporphyria,variegate porphyria,anderythropoietic protoporphyria.[32]
Synthesis for food
[edit]Impossible Foods,producers of plant-basedmeat substitutes,use an accelerated heme synthesis process involving soybean rootleghemoglobinandyeast,adding the resulting heme to items such as meatless (vegan) Impossible burger patties. The DNA forleghemoglobinproduction was extracted from the soybean root nodules and expressed in yeast cells to overproduce heme for use in the meatless burgers.[33]This process claims to create a meaty flavor in the resulting products.[34][35]
Degradation
[edit]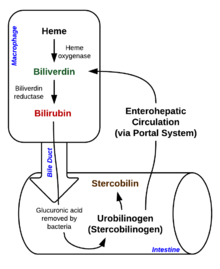
Degradation begins inside macrophages of thespleen,which remove old and damagederythrocytesfrom the circulation.
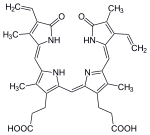
In the first step, heme is converted tobiliverdinby the enzymeheme oxygenase(HO).[36]NADPHis used as the reducing agent, molecular oxygen enters the reaction,carbon monoxide(CO) is produced and the iron is released from the molecule as theferrousion (Fe2+).[37]CO acts as a cellular messenger and functions in vasodilation.[38]
In addition, heme degradation appears to be an evolutionarily-conserved response tooxidative stress.Briefly, when cells are exposed tofree radicals,there is a rapid induction of the expression of the stress-responsiveheme oxygenase-1(HMOX1) isoenzyme that catabolizes heme (see below).[39]The reason why cells must increase exponentially their capability to degrade heme in response to oxidative stress remains unclear but this appears to be part of a cytoprotective response that avoids the deleterious effects of free heme. When large amounts of free heme accumulates, the heme detoxification/degradation systems get overwhelmed, enabling heme to exert its damaging effects.[31]
| heme | heme oxygenase-1 | biliverdin+ Fe2+ | |

|
| ||
| H++NADPH+ O2 | NADP++ CO | ||
| |||
In the second reaction,biliverdinis converted tobilirubinbybiliverdin reductase(BVR):[40]
| biliverdin | biliverdin reductase | bilirubin | |

|

| ||
| H++NADPH | NADP+ | ||

| |||
Bilirubin is transported into the liver by facilitated diffusion bound to a protein (serum albumin), where it is conjugated withglucuronic acidto become more water-soluble. The reaction is catalyzed by the enzyme UDP-glucuronosyltransferase.[41]
| bilirubin | UDP-glucuronosyltransferase | bilirubin diglucuronide | |

|

| ||
| 2UDP-glucuronide | 2UMP+ 2Pi | ||

| |||
This form of bilirubin is excreted from the liver inbile.Excretion of bilirubin from liver to biliary canaliculi is an active, energy-dependent and rate-limiting process. Theintestinal bacteriadeconjugatebilirubin diglucuronidereleasing free bilirubin, which can either be reabsorbed or reduced tourobilinogenby the bacterial enzyme bilirubin reductase.[42]
| bilirubin | bilirubin reductase | urobilinogen | |

|

| ||
| 4NAD(P)H+ 4 H+ | 4 NAD(P)+ | ||

| |||
Some urobilinogen is absorbed by intestinal cells and transported into thekidneysand excreted withurine(urobilin,which is the product of oxidation of urobilinogen, and is responsible for the yellow colour of urine). The remainder travels down the digestive tract and is converted tostercobilinogen.This is oxidized tostercobilin,which is excreted and is responsible for the brown color offeces.[43]
In health and disease
[edit]Underhomeostasis,the reactivity of heme is controlled by its insertion into the "heme pockets" of hemoproteins.[citation needed]Under oxidative stress however, some hemoproteins, e.g. hemoglobin, can release their heme prosthetic groups.[44][45]The non-protein-bound (free) heme produced in this manner becomes highly cytotoxic, most probably due to the iron atom contained within its protoporphyrin IX ring, which can act as aFenton's reagentto catalyze in an unfettered manner the production of free radicals.[46]It catalyzes the oxidation and aggregation of protein, the formation of cytotoxic lipid peroxide via lipid peroxidation and damages DNA through oxidative stress. Due to its lipophilic properties, it impairs lipid bilayers in organelles such as mitochondria and nuclei.[47]These properties of free heme can sensitize a variety of cell types to undergoprogrammed cell deathin response to pro-inflammatory agonists, a deleterious effect that plays an important role in the pathogenesis of certain inflammatory diseases such asmalaria[48]andsepsis.[49]
Cancer
[edit]There is an association between high intake of heme iron sourced from meat and increased risk ofcolorectal cancer.[50]
TheAmerican Institute for Cancer Research(AICR) and World Cancer Research Fund International (WCRF) concluded in a 2018 report that there is limited but suggestive evidence that foods containing heme iron increase risk of colorectal cancer.[51]A 2019 review found that heme iron intake is associated with increasedbreast cancerrisk.[52]
Genes
[edit]The following genes are part of the chemical pathway for making heme:
- ALAD:aminolevulinic acid, δ-,dehydratase(deficiency causes ala-dehydratase deficiency porphyria)[53]
- ALAS1:aminolevulinate, δ-, synthase 1
- ALAS2:aminolevulinate, δ-, synthase 2 (deficiency causes sideroblastic/hypochromic anemia)
- CPOX:coproporphyrinogenoxidase(deficiency causes hereditary coproporphyria)[54]
- FECH:ferrochelatase(deficiency causeserythropoietic protoporphyria)
- HMBS:hydroxymethylbilanesynthase(deficiency causes acute intermittent porphyria)[55]
- PPOX:protoporphyrinogenoxidase(deficiency causes variegate porphyria)[56]
- UROD:uroporphyrinogendecarboxylase(deficiency causes porphyria cutanea tarda)[57]
- UROS:uroporphyrinogenIIIsynthase(deficiency causes congenital erythropoietic porphyria)
Notes and references
[edit]- ^Hodgson E, Roe RM, Mailman RB, Chambers JE, eds. (2015)."H".Dictionary of Toxicology(3rd ed.). Academic Press. pp. 173–184.doi:10.1016/B978-0-12-420169-9.00008-4.ISBN978-0-12-420169-9.Retrieved2024-02-21.
- ^Bloomer JR (1998)."Liver metabolism of porphyrins and haem".Journal of Gastroenterology and Hepatology.13(3): 324–329.doi:10.1111/j.1440-1746.1998.01548.x.PMID9570250.S2CID25224821.
- ^Dutt S, Hamza I, Bartnikas TB (2022-08-22)."Molecular Mechanisms of Iron and Heme Metabolism".Annual Review of Nutrition.42(1): 311–335.doi:10.1146/annurev-nutr-062320-112625.ISSN0199-9885.PMC9398995.PMID35508203.
- ^Ogun AS, Joy NV, Valentine M (2024),"Biochemistry, Heme Synthesis",StatPearls,Treasure Island (FL): StatPearls Publishing,PMID30726014,retrieved2024-02-22
- ^Chemistry IU (2009)."Hemes (heme derivatives)".IUPAC Compendium of Chemical Terminology.IUPAC.doi:10.1351/goldbook.H02773.ISBN978-0-9678550-9-7.Archivedfrom the original on 22 August 2017.Retrieved28 April2018.
- ^A standard biochemistry text defines heme as the "iron-porphyrin prosthetic group of heme proteins" (Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000.ISBN1-57259-153-6.)
- ^Poulos TL (2014-04-09)."Heme Enzyme Structure and Function".Chemical Reviews.114(7): 3919–3962.doi:10.1021/cr400415k.ISSN0009-2665.PMC3981943.PMID24400737.
- ^Paoli M (2002)."Structure-function relationships in heme-proteins"(PDF).DNA Cell Biol.21(4): 271–280.doi:10.1089/104454902753759690.hdl:20.500.11820/67200894-eb9f-47a2-9542-02877d41fdd7.PMID12042067.S2CID12806393.Archived(PDF)from the original on 2018-07-24.
- ^Alderton W (2001)."Nitric oxide synthases: structure, function and inhibition".Biochem. J.357(3): 593–615.doi:10.1042/bj3570593.PMC1221991.PMID11463332.
- ^Milani M (2005). "Structural bases for heme binding and diatomic ligand recognition in truncated hemoglobins".J. Inorg. Biochem.99(1): 97–109.doi:10.1016/j.jinorgbio.2004.10.035.PMID15598494.
- ^Hardison R (1999). "The Evolution of Hemoglobin: Studies of a very ancient protein suggest that changes in gene regulation are an important part of the evolutionary story".American Scientist.87(2): 126.doi:10.1511/1999.20.809.S2CID123532036.
- ^Poulos T (2014)."Heme Enzyme Structure and Function".Chem. Rev.114(7): 3919–3962.doi:10.1021/cr400415k.PMC3981943.PMID24400737.
- ^Thom CS (2013)."Hemoglobin Variants: Biochemical Properties and Clinical Correlates".Cold Spring Harbor Perspectives in Medicine.3(3): a011858.doi:10.1101/cshperspect.a011858.PMC3579210.PMID23388674.
- ^Bohr, Hasselbalch, Krogh."Concerning a Biologically Important Relationship - The Influence of the Carbon Dioxide Content of Blood on its Oxygen Binding".Archivedfrom the original on 2017-04-18.
{{cite journal}}:Cite journal requires|journal=(help) - ^Ackers GK, Holt JM (2006)."Asymmetric cooperativity in a symmetric tetramer: human hemoglobin".J. Biol. Chem.281(17): 11441–3.doi:10.1074/jbc.r500019200.PMID16423822.S2CID6696041.
- ^Caughey WS, Smythe GE, O'Keeffe DH, Maskasky JE, Smith ML (1975)."Heme A of Cytochrome c Oxidase: Structure and properties: comparisons with hemes B, C, and S and derivatives".J. Biol. Chem.250(19): 7602–7622.doi:10.1016/S0021-9258(19)40860-0.PMID170266.
- ^Hegg EL (2004). "Heme A Synthase Does Not Incorporate Molecular Oxygen into the Formyl Group of Heme A".Biochemistry.43(27): 8616–8624.doi:10.1021/bi049056m.PMID15236569.
- ^Yoshikawa S (2012)."Structural studies on bovine heart cytochrome c oxidase".Biochim. Biophys. Acta.1817(4): 579–589.doi:10.1016/j.bbabio.2011.12.012.PMID22236806.
- ^Rae T, Goff H (1998)."The heme prosthetic group of lactoperoxidase. Structural characteristics of heme l and heme l-peptides".The Journal of Biological Chemistry.273(43): 27968–27977.doi:10.1074/jbc.273.43.27968.PMID9774411.S2CID25780396.
- ^Purdy M (1983)."Effect of growth phase and cell envelope structure on susceptibility of Salmonella triumphant to the lactoperoxidase-thiocyanate-hydrogen peroxide system".Infect. Immun.39(3): 1187–95.doi:10.1128/IAI.39.3.1187-1195.1983.PMC348082.PMID6341231.
- ^Ohshima H (2003). "Chemical basis of inflammation-induced carcinogenesis".Arch. Biochem. Biophys.417(1): 3–11.doi:10.1016/s0003-9861(03)00283-2.PMID12921773.
- ^Henderson J (2003)."Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue".J. Biol. Chem.278(26): 23522–8.doi:10.1074/jbc.m303928200.PMID12707270.S2CID19631565.
- ^Murshudov G, Grebenko A, Barynin V, Dauter Z, Wilson K, Vainshtein B, Melik-Adamyan W, Bravo J, Ferrán J, Ferrer JC, Switala J, Loewen PC, Fita I (1996)."Structure of the hemedofPenicillium vitaleandEscherichia colicatalases "(PDF).The Journal of Biological Chemistry.271(15): 8863–8868.doi:10.1074/jbc.271.15.8863.PMID8621527.Archived(PDF)from the original on 2018-07-24.
- ^Belevich I (2005). "Oxygenated complex of cytochrome bd from Escherichia coli: stability and photolability".FEBS Letters.579(21): 4567–70.doi:10.1016/j.febslet.2005.07.011.PMID16087180.S2CID36465802.
- ^Fischer H, Orth H (1934).Die Chemie des Pyrrols.Liepzig:Ischemia Verlagsgesellschaft.
- ^Puustinen A, Wikström M. (1991)."The heme groups of cytochrome o from Escherichia coli".Proc. Natl. Acad. Sci. U.S.A.88(14): 6122–6.Bibcode:1991PNAS...88.6122P.doi:10.1073/pnas.88.14.6122.PMC52034.PMID2068092.
- ^Caughey WS, York JL (1962)."Isolation and some properties of the green heme of cytochrome oxidase from beef heart muscle".J. Biol. Chem.237(7): 2414–6.doi:10.1016/S0021-9258(19)63456-3.PMID13877421.
- ^Caughey WS, Smythe GA, O'Keeffe DH, Maskasky JE, Smith ML (1975)."Heme A of cytochrome c oxidase. Structure and properties: comparisons with hemes B, C, and S and derivatives".J. Biol. Chem.250(19): 7602–22.doi:10.1016/S0021-9258(19)40860-0.PMID170266.
- ^Battersby AR (2000). "Tetrapyrroles: The pigments of life".Natural Product Reports.17(6): 507–526.doi:10.1039/B002635M.PMID11152419.
- ^Sridevi K (28 April 2018).Upregulation of Heme Pathway Enzyme ALA Synthase-1 by Glutethimide and 4,6-Dioxoheptanoic Acid and Downregulation by Glucose and Heme: A Dissertation.EScholarship@UMMS(Thesis). University of Massachusetts Medical School.doi:10.13028/yyrz-qa79.Archivedfrom the original on 8 August 2016.Retrieved28 April2018.
- ^abKumar S, Bandyopadhyay U (July 2005). "Free heme toxicity and its detoxification systems in human".Toxicology Letters.157(3): 175–188.doi:10.1016/j.toxlet.2005.03.004.PMID15917143.
- ^Puy H, Gouya L, Deybach JC (March 2010)."Porphyrias".The Lancet.375(9718): 924–937.doi:10.1016/S0140-6736(09)61925-5.PMID20226990.S2CID208791867.
- ^Fraser RZ, Shitut M, Agrawal P, Mendes O, Klapholz S (2018-04-11)."Safety Evaluation of Soy Leghemoglobin Protein Preparation Derived FromPichia pastoris, Intended for Use as a Flavor Catalyst in Plant-Based Meat".International Journal of Toxicology.37(3): 241–262.doi:10.1177/1091581818766318.ISSN1091-5818.PMC5956568.PMID29642729.
- ^"Inside the Strange Science of the Fake Meat That 'Bleeds'".Wired.2017-09-20.Archivedfrom the original on 24 March 2018.Retrieved28 April2018.
- ^"Silicon Valley gets a taste for food".The Economist.2015-03-05.ISSN0013-0613.Retrieved2019-04-08.
- ^Maines MD (July 1988)."Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications".The FASEB Journal.2(10): 2557–2568.doi:10.1096/fasebj.2.10.3290025.ISSN0892-6638.PMID3290025.S2CID22652094.
- ^Lehninger's Principles of Biochemistry(5th ed.). New York: W. H. Freeman and Company. 2008. pp.876.ISBN978-0-7167-7108-1.
- ^Hanafy K (2013)."Carbon Monoxide and the brain: time to rethink the dogma".Curr. Pharm. Des.19(15): 2771–5.doi:10.2174/1381612811319150013.PMC3672861.PMID23092321.
- ^Abraham N, Kappas A (2008). "Pharmacological and clinical aspects of heme oxygenase".Pharmacol. Rev.60(1): 79–127.doi:10.1124/pr.107.07104.PMID18323402.S2CID12792155.
- ^Florczyk U, Jozkowicz A, Dulak J (January–February 2008)."Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance".Pharmacological Reports.60(1): 38–48.PMC5536200.PMID18276984.
- ^King C, Rios G, Green M, Tephly T (2000). "UDP-Glucuronosyltransferases".Current Drug Metabolism.1(2): 143–161.doi:10.2174/1389200003339171.PMID11465080.
- ^Hall B, Levy S, Dufault-Thompson K, Arp G, Zhong A, Ndjite GM, Weiss A, Braccia D, Jenkins C, Grant MR, Abeysinghe S, Yang Y, Jermain MD, Wu CH, Ma B (2024-01-03)."BilR is a gut microbial enzyme that reduces bilirubin to urobilinogen".Nature Microbiology.9(1): 173–184.doi:10.1038/s41564-023-01549-x.ISSN2058-5276.PMC10769871.PMID38172624.
- ^Helmenstine AM."The Chemicals Responsible for the Color of Urine and Feces".ThoughtCo.Retrieved2020-01-24.
- ^Bunn HF, Jandl JH (Sep 1966)."Exchange of heme among hemoglobin molecules".Proc. Natl. Acad. Sci. USA.56(3): 974–978.Bibcode:1966PNAS...56..974B.doi:10.1073/pnas.56.3.974.PMC219955.PMID5230192.
- ^Smith ML, Paul J, Ohlsson PI, Hjortsberg K, Paul KG (Feb 1991)."Heme-protein fission under nondenaturing conditions".Proc. Natl. Acad. Sci. USA.88(3): 882–886.Bibcode:1991PNAS...88..882S.doi:10.1073/pnas.88.3.882.PMC50918.PMID1846966.
- ^Everse J, Hsia N (1197). "The toxicities of native and modified hemoglobins".Free Radical Biology and Medicine.22(6): 1075–1099.doi:10.1016/S0891-5849(96)00499-6.PMID9034247.
- ^Kumar S, Bandyopadhyay U (July 2005). "Free heme toxicity and its detoxification systems in humans".Toxicology Letters.157(3): 175–188.doi:10.1016/j.toxlet.2005.03.004.PMID15917143.
- ^Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP, Mota MM (Jun 2007). "Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria".Nature Medicine.13(6): 703–710.doi:10.1038/nm1586.PMID17496899.S2CID20675040.
- ^Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP (2010)."A central role for free heme in the pathogenesis of severe sepsis".Science Translational Medicine.2(51): 51ra71.doi:10.1126/scitranslmed.3001118.PMID20881280.S2CID423446.
- ^Bastide NM, Pierre FH, Corpet DE (Feb 2011)."Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved".Cancer Prevention Research (Philadelphia, Pa.).4(2): 177–184.doi:10.1158/1940-6207.CAPR-10-0113.ISSN1940-6215.PMID21209396.
- ^"Diet, nutrition, physical activity and colorectal cancer".wcrf.org. Retrieved 12 February 2022.
- ^Chang, Vicky C; Cotterchio, Michelle; Khoo, Edwin (2019)."Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis".BMC Cancer.19(1): 543.doi:10.1186/s12885-019-5642-0.PMC6555759.PMID31170936.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Plewinska M, Thunell S, Holmberg L, Wetmur J, Desnick R (1991)."delta-Aminolevulinate dehydratase deficient porphyria: identification of the molecular lesions in a severely affected homozygote".American Journal of Human Genetics.49(1): 167–174.PMC1683193.PMID2063868.
- ^Aurizi C, Lupia Palmieri G, Barbieri L, Macri A, Sorge F, Usai G, Biolcati G (February 2009). "Four novel mutations of the coproporphyrinogen III oxidase gene".Cellular and Molecular Biology.55(1): 8–15.PMID19267996.
- ^Bustad HJ, Vorland M, Ronneseth E, Sandberg S, Martinez A, Toska K (August 8, 2013)."Conformational stability and activity analysis of two hydroxymethylbilane synthase mutants, K132N and V215E, with different phenotypic association with acute intermittent porphyria".Bioscience Reports.33(4): 617–626.doi:10.1042/BSR20130045.PMC3738108.PMID23815679.
- ^Martinez di Montemuros F, Di Pierro E, Patti E, Tavazzi D, Danielli MG, Biolcati G, Rocchi E, Cappellini MD (December 2002)."Molecular characterization of porphyrias in Italy: a diagnostic flow-chart".Cellular and Molecular Biology (Noisy-Le-Grand, France).48(8): 867–876.ISSN0145-5680.PMID12699245.
- ^Badenas C, To Figueras J, Phillips JD, Warby CA, Muñoz C, Herrero C (April 2009)."Identification and characterization of novel uroporphyrinogen decarboxylase gene mutations in a large series of porphyria cutanea tarda patients and relatives".Clinical Genetics.75(4): 346–353.doi:10.1111/j.1399-0004.2009.01153.x.PMC3804340.PMID19419417.
