Heterocyclic compound
Aheterocyclic compoundorring structureis acyclic compoundthat has atoms of at least two differentelementsas members of its ring(s).[1]Heterocyclic organic chemistryis the branch oforganic chemistrydealing with the synthesis, properties, and applications oforganic heterocycles.[2]
Examples of heterocyclic compounds include all of thenucleic acids,the majority of drugs, mostbiomass(celluloseand related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles.[3]59% of USFDA-approved drugs containnitrogenheterocycles.[4]
Classification
[edit]The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included arepyridine,thiophene,pyrrole,andfuran.Another large class of organic heterocycles refers to those fused tobenzene rings.For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan arequinoline,benzothiophene,indole,andbenzofuran,respectively. The fusion of two benzene rings gives rise to a third large family of organic compounds. Analogs of the previously mentioned heterocycles for this third family of compounds areacridine,dibenzothiophene,carbazole,anddibenzofuran,respectively.
Heterocyclic organic compounds can be usefully classified based on their electronic structure. The saturated organic heterocycles behave like the acyclic derivatives. Thus,piperidineandtetrahydrofuranare conventionalaminesandethers,with modified steric profiles. Therefore, the study of organic heterocyclic chemistry focuses on organic unsaturated rings.
Inorganic rings
[edit]Some heterocycles contain no carbon. Examples areborazine(B3N3ring),hexachlorophosphazenes(P3N3rings), andtetrasulfur tetranitrideS4N4.In comparison with organic heterocycles, which have numerous commercial applications, inorganic ring systems are mainly of theoretical interest.IUPACrecommends theHantzsch-Widman nomenclaturefor naming heterocyclic compounds.[5]
Notes on lists
[edit]- "Heteroatoms" are atoms in the ring other thancarbonatoms.
- Names in italics are retained byIUPACand do not follow theHantzsch-Widman nomenclature
- Some of the names refer to classes of compounds rather than individual compounds.
- Also no attempt is made to listisomers.
3-membered rings
[edit]Although subject toring strain,3-membered heterocyclic rings are well characterized.[6]
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Boron | Borirane | Borirene |
| Nitrogen | Aziridine | Azirine |
| Oxygen | Oxirane(ethylene oxide,epoxides) | Oxirene |
| Phosphorus | Phosphirane | Phosphirene |
| Sulfur | Thiirane(episulfides) | Thiirene |
| Heteroatoms | Saturated | Unsaturated |
|---|---|---|
| 2× Nitrogen | Diaziridine | Diazirine |
| Nitrogen + oxygen | Oxaziridine | Oxazirine |
| 2× Oxygen | Dioxirane (highly unstable) |
4-membered rings
[edit]| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Azetidine | Azete |
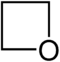
| Oxygen | Oxetane | Oxete |
| Phosphorus | Phosphetane | Phosphete |
| Sulfur | Thietane | Thiete |
| Heteroatoms | Saturated | Unsaturated |
|---|---|---|
| 2× Nitrogen | Diazetidine | Diazete |
| 2× Oxygen | Dioxetane | Dioxete |
| 2× Sulfur | Dithietane | Dithiete |
5-membered rings
[edit]The 5-membered ring compounds containingtwoheteroatoms, at least one of which is nitrogen, are collectively called theazoles.Thiazolesandisothiazolescontain asulfurand a nitrogen atom in the ring.Dithiolaneshave two sulfur atoms.
A large group of 5-membered ring compounds withthreeor more heteroatoms also exists. One example is the class ofdithiazoles,which contain two sulfur atoms and one nitrogen atom.
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Antimony | Stibolane | Stibole |
| Arsenic | Arsolane | Arsole |
| Bismuth | Bismolane | Bismole |
| Boron | Borolane | Borole |
| Nitrogen | Pyrrolidine( "Azolidine" not used) | Pyrrole( "Azole" not used) |
| Oxygen | Tetrahydrofuran | Furan |
| Phosphorus | Phospholane | Phosphole |
| Selenium | Selenolane | Selenophene |
| Silicon | Silacyclopentane | Silole |
| Sulfur | Tetrahydrothiophene | Thiophene |
| Tellurium | Tellurophene | |
| Tin | Stannolane | Stannole |
| Heteroatoms | Saturated | Unsaturated (and partially unsaturated) |
|---|---|---|
| 2× nitrogen | Imidazolidine Pyrazolidine |
Imidazole(Imidazoline) Pyrazole(Pyrazoline) |
| Oxygen + sulfur | 1,3-Oxathiolane 1,2-Oxathiolane |
Oxathiole(Oxathioline) Isoxathiole |
| Nitrogen + Oxygen | Oxazolidine Isoxazolidine |
Oxazole(Oxazoline) Isoxazole |
| Nitrogen + sulfur | Thiazolidine Isothiazolidine |
Thiazole(Thiazoline) Isothiazole |
| 2× oxygen | Dioxolane | |
| 2× sulfur | Dithiolane | Dithiole |
| Heteroatoms | Saturated | Unsaturated |
|---|---|---|
| N N N | Triazoles | |
| N N O | Furazan Oxadiazole | |
| N N S | Thiadiazole | |
| N O O | Dioxazole | |
| N S S | Dithiazole | |
| N N N N | Tetrazole | |
| N N N N O | Oxatetrazole | |
| N N N N S | Thiatetrazole | |
| N N N N N | Pentazole |
6-membered rings
[edit]| Heteroatom | Saturated | Unsaturated | Ions |
|---|---|---|---|
| Antimony | Stibinin[7] | ||
| Arsenic | Arsinane | Arsinine | |
| Bismuth | Bismin[8] | ||
| Boron | Borinane | Borinine | Boratabenzeneanion |
| Germanium | Germinane | Germine | |
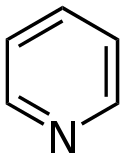
| Nitrogen | Piperidine (Azinane not used) |
Pyridine (Azinenot used) |
Pyridiniumcation |
| Oxygen | Oxane | Pyran (2H-Oxinenot used) |
Pyryliumcation |
| Phosphorus | Phosphinane | Phosphinine | |
| Selenium | Selenane | Selenopyran[9] | Selenopyryliumcation |
| Silicon | Silinane | Siline | |
| Sulfur | Thiane | Thiopyran (2H-Thiine not used) |
Thiopyryliumcation |
| Tellurium | Tellurane | Telluropyran | Telluropyryliumcation |
| Tin | Stanninane | Stannine |
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen / nitrogen | Diazinane | Diazine |
| Oxygen / nitrogen | Morpholine | Oxazine |
| Sulfur / nitrogen | Thiomorpholine | Thiazine |
| Oxygen / Sulfur | Oxathiane | Oxathiin |
| Oxygen / oxygen | Dioxane | Dioxine |
| Sulfur / sulfur | Dithiane | Dithiin |
| Boron / nitrogen | 1,2-Dihydro-1,2-azaborine |
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Triazinane | Triazine |
| Oxygen | Trioxane | |
| Sulfur | Trithiane |
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Tetrazine | |
| 2 nitrogen, 2boron | Carborazine |
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Pentazine |
Six-membered rings with six heteroatoms
[edit]Thehypothetical chemical compoundwith six nitrogen heteroatoms would behexazine.Borazineis a six-membered ring with three nitrogen heteroatoms and three boron heteroatoms.
7-membered rings
[edit]In a 7-membered ring, the heteroatom must be able to provide an empty π-orbital (e.g. boron) for "normal" aromatic stabilization to be available; otherwise,homoaromaticitymay be possible.
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Boron | Borepin | |
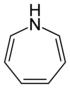
| Nitrogen | Azepane | Azepine |
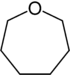
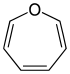
| Oxygen | Oxepane | Oxepine |
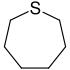
| Sulfur | Thiepane | Thiepine |
| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Diazepane | Diazepine |
| Nitrogen/sulfur | Thiazepine |
8-membered rings
[edit]| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Azocane | Azocine |
| Oxygen | Oxocane | Oxocine |
| Sulfur | Thiocane | Thiocine |
| 4 nitrogen, 4 boron | Borazocine |
9-membered rings
[edit]| Heteroatom | Saturated | Unsaturated |
|---|---|---|
| Nitrogen | Azonane | Azonine |
| Oxygen | Oxonane | Oxonine |
| Sulfur | Thionane | Thionine |
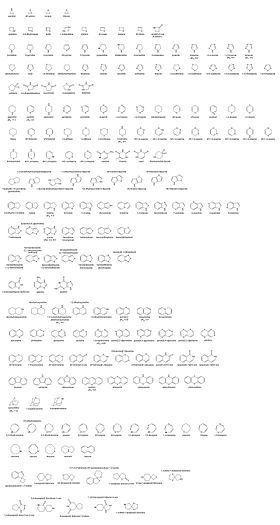
Images of rings with one heteroatom
[edit]| Saturated | Unsaturated | ||||||
|---|---|---|---|---|---|---|---|
| Heteroatom | Nitrogen | Oxygen | Sulfur | Nitrogen | Oxygen | Sulfur | |
| 3-atom ring | Aziridine | Oxirane | Thiirane | Azirine | Oxirene | Thiirene | |
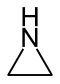 |
 |
 |
 |
 |

| ||
| 4-atom ring | Azetidine | Oxetane | Thietane | Azete | Oxete | Thiete | |
 |
 |
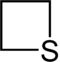 |
 |
 |

| ||
| 5-atom ring | Pyrrolidine | Oxolane | Thiolane | Pyrrole | Furan | Thiophene | |
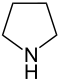 |
 |

|
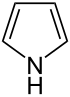 |
 |
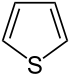
| ||
| 6-atom ring | Piperidine | Oxane | Thiane | Pyridine | Pyran | Thiopyran | |
 |
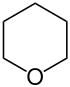 |
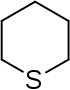
|
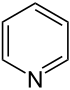 |
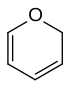 |

| ||
| 7-atom ring | Azepane | Oxepane | Thiepane | Azepine | Oxepine | Thiepine | |
 |
 |
 |
 |
 |
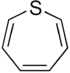
| ||
| 8-atom ring | Azocane | Oxocane | Thiocane | Azocine | Oxocine | Thiocine | |
 |
 |
 |
 |
 |

| ||
| 9-atom ring | Azonane | Oxonane | Thionane | Azonine | Oxonine | Thionine | |
 |
 |
 |
 |
 |

| ||
Fused/condensed rings
[edit]Heterocyclic rings systems that are formally derived by fusion with other rings, eithercarbocyclicor heterocyclic, have a variety of common and systematic names. For example, with the benzo-fused unsaturated nitrogen heterocycles, pyrrole providesindoleorisoindoledepending on the orientation. The pyridine analog isquinolineorisoquinoline.For azepine,benzazepineis the preferred name. Likewise, the compounds with two benzene rings fused to the central heterocycle arecarbazole,acridine,and dibenzoazepine.Thienothiopheneare the fusion of two thiophene rings.Phosphaphenalenesare a tricyclic phosphorus-containing heterocyclic system derived from the carbocyclephenalene.
History of heterocyclic chemistry
[edit]The history of heterocyclic chemistry began in the 1800s, in step with the development oforganic chemistry.Some noteworthy developments:[10]
- 1818: Brugnatelli makesalloxanfromuric acid
- 1832: Dobereiner producesfurfural(a furan) by treatingstarchwithsulfuric acid
- 1834: Runge obtainspyrrole( "fiery oil" ) by dry distillation of bones
- 1906: Friedlander synthesizesindigo dye,allowing synthetic chemistry to displace a large agricultural industry
- 1936:Treibsisolates chlorophyll derivatives from crude oil, explaining the biological origin of petroleum.
- 1951:Chargaff's rulesare described, highlighting the role of heterocyclic compounds (purinesandpyrimidines) in the genetic code.
Uses
[edit]Heterocyclic compounds are pervasive in many areas of life sciences and technology.[2]Many drugs are heterocyclic compounds.[11]
See also
[edit]References
[edit]- ^IUPAC Gold Bookheterocyclic compounds
- ^abThomas L. Gilchrist "Heterocyclic Chemistry" 3rd ed. Addison Wesley: Essex, England, 1997. 414 pp.ISBN0-582-27843-0.
- ^Rees, Charles W. (1992). "Polysulfur-Nitrogen Heterocyclic Chemistry".Journal of Heterocyclic Chemistry.29(3): 639–651.doi:10.1002/jhet.5570290306.
- ^Edon Vitaku, David T. Smith, Jon T. Njardarson (2014). "Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals".J. Med. Chem.57(24): 10257–10274.doi:10.1021/jm501100b.PMID25255204.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "Hantzsch–Widman name".doi:10.1351/goldbook.H02737
- ^Smith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience,ISBN978-0-471-72091-1
- ^"Stibinin".chemspider.Royal Society of Chemistry.Retrieved11 June2018.
- ^"Bismin".ChemSpider.Royal Society of Chemistry.Retrieved11 June2018.
- ^"Selenopyranium".ChemSpider.Royal Society of Chemistry.Retrieved11 June2018.
- ^Campaigne, E. (1986). "Adrien Albert and the rationalization of heterocyclic chemistry".Journal of Chemical Education.63(10): 860.Bibcode:1986JChEd..63..860C.doi:10.1021/ed063p860.
- ^"IPEXL Multilingual Patent Search, Patent Ranking".ipexl.Archived fromthe originalon 24 September 2015.Retrieved8 September2010.
