Housane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[2.1.0]pentane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChemCID
|
|||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.119g·mol−1 | ||
| Appearance | colorless liquid | ||
| Boiling point | 45.5 ′C | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
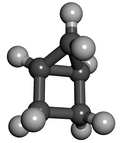
Housaneorbicyclo[2.1.0]pentaneis a saturatedcycloalkanewith the formula C5H8.It is a colorless, volatile liquid at room temperature. It was named "housane" because of its shape, which resembles a simple drawing of a house. Structurally, the molecule consists ofcyclopropanefused tocyclobutane.The synthesis of molecules containing multiple strained rings, such as housane, is a traditional endeavor insynthetic organic chemistry.
Preparation
[edit]The first synthesis of housane was reported byCriegeein 1957, where housane was obtained from thepyrolysisof 2,3-diazabicyclo[2.2.1]hept-2-ene.[1]
Housane can be prepared in several steps starting withcyclopentadiene.Other methods include photolysis of 2,3-diazabicyclo[2.2.1]hept-2-ene, pyrolysis ofN-Phenyl-2-oxo-3-azabicyclo[2.2.1]heptane, and addition ofmethylenetocyclobutene.[2]
Structure and properties
[edit]- The two rings are fused in acisconfiguration—thismeso compoundformally has (1R,4S)absolute stereochemistry.The small size of the two rings prevents thetransisomer from existing, so the stereochemistry is not usually mentioned when discussing this structure.
Housane is easily attacked bybromineoriodine.In the presence of a platinum catalyst at room temperature, it is hydrogenated intocyclopentane.Reaction withhydrogen bromideat lower temperatures affordsbromocyclopentane.Housane also reacts withlead tetraacetate,formingcis-1,3-diacetoxycyclopentane among other products.[1]
Housane is thermally quite stable, isomerizing to cyclopentene at 330 °C.[1]
See also
[edit]References
[edit]- ^abcCriegee, Rudolf; Rimmelin, Annemarie (1957)."Darstellung und Eigenschaften von Bicyclo‐[0.1.2]‐Pentan"[Preparation and Properties of Bicyclo-[0.1.2]-pentane].Chemische Berichte.90(3): 414–417.doi:10.1002/cber.19570900319.ISSN0009-2940.
- ^P. G. Gassman, K. T. Mansfield "Bicyclo[2.1.0]pentane" Org. Synth. 1969, volume 49, pp. 1.doi:10.15227/orgsyn.049.0001