Hydrazine
| |||
| |||
 Anhydrous hydrazine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hydrazine[2]
| |||
| Systematic IUPAC name
Diazane[2] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 878137 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.560 | ||
| EC Number |
| ||
| 190 | |||
| KEGG | |||
| MeSH | Hydrazine | ||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2029 | ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| N2H4 | |||
| Molar mass | 32.0452 g/mol | ||
| Appearance | Colorless, fuming, oily liquid[3] | ||
| Odor | Ammonia-like[3] | ||
| Density | 1.021 g/cm3 | ||
| Melting point | 2 °C; 35 °F; 275 K | ||
| Boiling point | 114 °C; 237 °F; 387 K | ||
| Miscible[3] | |||
| logP | 0.67 | ||
| Vapor pressure | 1 kPa (at 30.7 °C) | ||
| Acidity(pKa) | 8.10 ([N2H5]+)[4] | ||
| Basicity(pKb) | 5.90 | ||
| Conjugate acid | Hydrazinium | ||
Refractive index(nD)
|
1.46044 (at 22 °C) | ||
| Viscosity | 0.876 cP | ||
| Structure | |||
| Triangular pyramidal at N | |||
| 1.85 D[5] | |||
| Thermochemistry | |||
Std molar
entropy(S⦵298) |
121.52 J/(K·mol) | ||
Std enthalpy of
formation(ΔfH⦵298) |
50.63 kJ/mol | ||
| Hazards | |||
| GHSlabelling: | |||
    
| |||
| Danger | |||
| H226,H301,H311,H314,H317,H331,H350,H410 | |||
| P201,P261,P273,P280,P301+P310,P305+P351+P338 | |||
| NFPA 704(fire diamond) | |||
| Flash point | 52 °C (126 °F; 325 K) | ||
| 24 to 270 °C (75 to 518 °F; 297 to 543 K) | |||
| Explosive limits | 1.8–100% | ||
| Lethal doseor concentration (LD, LC): | |||
LD50(median dose)
|
59–60 mg/kg (oral in rats, mice)[6] | ||
LC50(median concentration)
|
260 ppm (rat, 4h) 630 ppm (rat, 1 h) 570 ppm (rat, 4 h) 252 ppm (mouse, 4 h)[7] | ||
| NIOSH(US health exposure limits): | |||
PEL(Permissible)
|
TWA 1 ppm (1.3 mg/m3) [skin][3] | ||
REL(Recommended)
|
Ca C 0.03 ppm (0.04 mg/m3) [2-hour][3] | ||
IDLH(Immediate danger)
|
Ca [50 ppm][3] | ||
| Safety data sheet(SDS) | ICSC 0281 | ||
| Related compounds | |||
Otheranions
|
Tetrafluorohydrazine Hydrogen peroxide Diphosphane Diphosphorus tetraiodide | ||
Othercations
|
Organic hydrazines | ||
Related Binaryazanes
|
Ammonia Triazane | ||
Related compounds
|
Diazene Triazene Tetrazene Diphosphene | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Hydrazineis aninorganic compoundwith thechemical formulaN2H4.It is a simplepnictogen hydride,and is a colourless flammable liquid with anammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example,hydrazine hydrate(N2H4·xH2O).
Hydrazine is mainly used as afoaming agentin preparingpolymer foams,but applications also include its uses as aprecursortopharmaceuticalsandagrochemicals,as well as a long-termstorable propellantfor in-spacespacecraft propulsion. Additionally, hydrazine is used in variousrocket fuelsand to prepare the gas precursors used inair bags.Hydrazine is used within both nuclear and conventional electricalpower plantsteam cycles as anoxygen scavengerto control concentrations of dissolved oxygen in an effort to reduce corrosion.[8] As of 2000[update],approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) were manufactured worldwide per year.[9]
Hydrazinesare a class of organic substances derived by replacing one or more hydrogen atoms in hydrazine by an organic group.[9]
Etymology[edit]
The nomenclature is a bi-valent form, with prefixhydr-used to indicate the presence ofhydrogenatoms and suffix beginning with-az-,fromazote,the French word fornitrogen.
Applications[edit]
Gas producers and propellants[edit]
The largest use of hydrazine is as a precursor toblowing agents.Specific compounds includeazodicarbonamideandazobisisobutyronitrile,which produce100–200 mLof gas per gram of precursor. In a related application,sodium azide,the gas-forming agent inair bags,is produced from hydrazine by reaction withsodium nitrite.[9]
Hydrazine is also used as a long-termstorable propellanton boardspacevehicles, such as theDawnmission to Ceres and Vesta, and to both reduce the concentration of dissolved oxygen in and control pH of water used in large industrial boilers. TheF-16fighter jet,Eurofighter Typhoon,[10]Space Shuttle,andU-2spy plane use hydrazine to fuel their Emergency Start System in the event of an engine stall.[11]
Precursor to pesticides and pharmaceuticals[edit]

Hydrazine is a precursor to several pharmaceuticals and pesticides. Often these applications involve conversion of hydrazine toheterocyclic ringssuch aspyrazolesandpyridazines.Examples of commercialized bioactivehydrazine derivativesincludecefazolin,rizatriptan,anastrozole,fluconazole,metazachlor, metamitron,metribuzin,paclobutrazol,diclobutrazole,propiconazole,hydrazine sulfate,[12]diimide,triadimefon,[9]anddibenzoylhydrazine.
Hydrazine compounds can be effective as active ingredients in insecticides, miticides,nematicides,fungicides, antiviral agents, attractants, herbicides, or plant growth regulators.[13]
Small-scale, niche, and research[edit]
The Italiancatalystmanufacturer Acta (chemical company) has proposed using hydrazine as an alternative tohydrogeninfuel cells.The chief benefit of using hydrazine is that it can produce over 200 mW/cm2more than a similar hydrogen cell without requiring (expensive)platinumcatalysts.[14]Because the fuel is liquid at room temperature, it can be handled and stored more easily than hydrogen. By storing the hydrazine in a tank full of a double-bondedcarbon-oxygencarbonyl,the fuel reacts and forms a safe solid calledhydrazone.By then flushing the tank with warm water, the liquid hydrazine hydrate is released. Hydrazine has a higherelectromotive forceof 1.56Vcompared to 1.23 V for hydrogen. Hydrazine breaks down in the cell to formnitrogenandhydrogenwhich bonds with oxygen, releasing water.[14]Hydrazine was used in fuel cells manufactured byAllis-Chalmers Corp.,including some that provided electric power in space satellites in the 1960s.
A mixture of 63% hydrazine, 32%hydrazine nitrateand 5% water is a standard propellant for experimentalbulk-loaded liquid propellant artillery.The propellant mixture above is one of the most predictable and stable, with a flat pressure profile during firing. Misfires are usually caused by inadequate ignition. The movement of the shell after a mis-ignition causes a large bubble with a larger ignition surface area, and the greater rate of gas production causes very high pressure, sometimes including catastrophic tube failures (i.e. explosions).[15]From January–June 1991, theU.S. Army Research Laboratoryconducted a review of early bulk-loaded liquid propellant gun programs for possible relevance to the electrothermal chemical propulsion program.[15]
TheUnited States Air Force(USAF) regularly uses H-70, a 70% hydrazine 30% water mixture, in operations employing theGeneral Dynamics F-16 "Fighting Falcon"fighter aircraft and theLockheed U-2 "Dragon Lady"reconnaissance aircraft. The single jet engine F-16 utilizes hydrazine to power its Emergency Power Unit (EPU), which provides emergency electrical and hydraulic power in the event of an engine flame out. The EPU activates automatically, or manually by pilot control, in the event of loss of hydraulic pressure or electrical power in order to provide emergency flight controls. The single jet engine U-2 utilizes hydrazine to power its Emergency Starting System (ESS), which provides a highly reliable method to restart the engine in flight in the event of a stall.[16]
Rocket fuel[edit]

Hydrazine was first used as a component inrocket fuelsduringWorld War II.A 30% mix by weight with 57%methanol(namedM-Stoffin the GermanLuftwaffe) and 13% water was calledC-Stoffby the Germans.[17]The mixture was used to power theMesserschmitt Me 163Brocket-powered fighter plane, in which the Germanhigh test peroxideT-Stoffwas used as an oxidizer. Unmixed hydrazine was referred to asB-Stoffby the Germans, a designation also used later for the ethanol/water fuel for theV-2 missile.[18]
Hydrazine is used as a low-powermonopropellantfor the maneuvering (RCS/Reaction control system) thrusters of spacecraft, and was used to power theSpace Shuttle's auxiliary power units (APUs). In addition, mono-propellant hydrazine-fueled rocket engines are often used in terminal descent of spacecraft. Such engines were used on theViking programlanders in the 1970s as well as the Mars landersPhoenix(May 2008),Curiosity(August 2012), andPerseverance(February 2021).
A mixture of hydrazine andred fuming nitric acid(HNO3+ N2H4) was used as liquid rocket fuel during theSoviet space program,where it became known as "devil's venom"due to its highly dangerous nature.[19]
In all hydrazine mono-propellant engines, the hydrazine is passed over acatalystsuch asiridiummetal supported by high-surface-areaalumina(aluminium oxide), which causes it to decompose intoammonia(NH3), nitrogen gas (N2), and hydrogen (H2) gas according to the three following reactions:[20]
- Reaction 1: N2H4→ N2+ 2 H2
- Reaction 2: 3 N2H4→ 4 NH3+ N2
- Reaction 3: 4 NH3+ N2H4→ 3 N2+ 8 H2
The first two reactions are extremelyexothermic(the catalyst chamber can reach 800 °C in a matter of milliseconds,[21]) and they produce large volumes of hot gas from a small volume of liquid,[22]making hydrazine a fairly efficient thruster propellant with a vacuumspecific impulseof about 220 seconds.[23]Reaction 2 is the most exothermic, but produces a smaller number of molecules than that of reaction 1. Reaction 3 isendothermicand reverts the effect of reaction 2 back to the same effect as reaction 1 alone (lower temperature, greater number of molecules). The catalyst structure affects the proportion of theNH3that is dissociated in reaction 3; a higher temperature is desirable for rocket thrusters, while more molecules are desirable when the reactions are intended to produce greater quantities of gas.[24]
Since hydrazine is a solid below 2 °C, it is not suitable as a general purpose rocket propellant for military applications. Othervariants of hydrazinethat are used as rocket fuel aremonomethylhydrazine,CH3NHNH2,also known as MMH (melting point −52 °C), andunsymmetrical dimethylhydrazine,(CH3)2NNH2,also known as UDMH (melting point −57 °C). These derivatives are used in two-component rocket fuels, often together withdinitrogen tetroxide,N2O4.A 50:50 mixture by weight of hydrazine and UDMH was used in the engine of the service propulsion system of theApollo command and service module,both the ascent and descent engines of theApollo Lunar ModuleandTitan IIICBMsand is known asAerozine 50.[17]These reactions are extremely exothermic, and the burning is alsohypergolic(it starts burning without any external ignition).[25]
In the fictional bookThe Martian(also adopted to afeature film) the titular character uses aniridiumcatalyst to separatehydrogengas from surplus hydrazine fuel, which he then burns to generate water for survival.
There are ongoing efforts in the aerospace industry to find a replacement for hydrazine, given its potential ban across the European Union.[26][27][28]Promising alternatives includenitrous oxide-based propellant combinations, with development being led by commercial companiesDawn Aerospace,Impulse Space,[29]andLauncher.[30]The first nitrous oxide-based system ever flown in space was byD-Orbitonboard theirION Satellite Carrierin 2021, using six Dawn Aerospace B20 thrusters.[31][32]
Occupational hazards[edit]
Health effects[edit]
Potential routes of hydrazine exposure include dermal, ocular, inhalation and ingestion.[33]
Hydrazine exposure can cause skin irritation/contact dermatitis and burning, irritation to the eyes/nose/throat, nausea/vomiting, shortness of breath, pulmonary edema, headache, dizziness, central nervous system depression, lethargy, temporary blindness, seizures and coma. Exposure can also cause organ damage to the liver, kidneys and central nervous system.[33][34]Hydrazine is documented as a strong skin sensitizer with potential for cross-sensitization to hydrazine derivatives following initial exposure.[35]In addition to occupational uses reviewed above, exposure to hydrazine is also possible in small amounts from tobacco smoke.[34]
The official U.S. guidance on hydrazine as a carcinogen is mixed but generally there is recognition of potential cancer-causing effects. TheNational Institute for Occupational Safety and Health (NIOSH)lists it as a "potential occupational carcinogen". The National Toxicology Program (NTP) finds it is "reasonably anticipated to be a human carcinogen". TheAmerican Conference of Governmental Industrial Hygienists (ACGIH)grades hydrazine as "A3—confirmed animal carcinogen with unknown relevance to humans". The U.S. Environmental Protection Agency (EPA) grades it as "B2—a probable human carcinogen based on animal study evidence".[36]
The International Agency for Research on Cancer (IARC) rates hydrazine as "2A—probably carcinogenic to humans" with a positive association observed between hydrazine exposure and lung cancer.[37]Based on cohort and cross-sectional studies of occupational hydrazine exposure, a committee from theNational Academies of Sciences,Engineering and Medicine concluded that there is suggestive evidence of an association between hydrazine exposure and lung cancer, with insufficient evidence of association with cancer at other sites.[38]TheEuropean Commission'sScientific Committee on Occupational Exposure Limits(SCOEL) places hydrazine in carcinogen "group B—a genotoxic carcinogen". The genotoxic mechanism the committee cited references hydrazine's reaction with endogenous formaldehyde and formation of a DNA-methylating agent.[39]
In the event of a hydrazine exposure-related emergency,NIOSHrecommends removing contaminated clothing immediately, washing skin with soap and water, and for eye exposure removing contact lenses and flushing eyes with water for at least 15 minutes.NIOSHalso recommends anyone with potential hydrazine exposure to seek medical attention as soon as possible.[33]There are no specific post-exposure laboratory or medical imaging recommendations, and the medical work-up may depend on the type and severity of symptoms. TheWorld Health Organization(WHO) recommends potential exposures be treated symptomatically with special attention given to potential lung and liver damage. Past cases of hydrazine exposure have documented success with pyridoxine (vitamin B6) treatment.[35]
Occupational exposure limits[edit]
- NIOSHRecommended Exposure Limit (REL): 0.03ppm(0.04 mg/m3) 2-hour ceiling[36]
- OSHAPermissible Exposure Limit (PEL): 1 ppm (1.3 mg/m3) 8-hour Time Weighted Average[36]
- ACGIHThreshold Limit Value (TLV): 0.01 ppm (0.013 mg/m3) 8-hour Time Weighted Average[36]
The odor threshold for hydrazine is 3.7 ppm, thus if a worker is able to smell an ammonia-like odor then they are likely over the exposure limit. However, this odor threshold varies greatly and should not be used to determine potentially hazardous exposures.[40]
For aerospace personnel, theUnited States Air Forceuses an emergency exposure guideline, developed by theNational Academy of SciencesCommittee on Toxicology, which is utilized for non-routine exposures of the general public and is called the Short-Term Public Emergency Exposure Guideline (SPEGL). The SPEGL, which does not apply to occupational exposures, is defined as the acceptable peak concentration for unpredicted, single, short-term emergency exposures of the general public and represents rare exposures in a worker's lifetime. For hydrazine the 1-hour SPEGL is 2 ppm, with a 24-hour SPEGL of 0.08 ppm.[41]
Handling and medical surveillance[edit]
A complete surveillance programme for hydrazine should include systematic analysis of biologic monitoring, medical screening and morbidity/mortality information. TheCDCrecommends surveillance summaries and education be provided for supervisors and workers. Pre-placement and periodic medical screening should be conducted with specific focus on potential effects of hydrazine upon functioning of the eyes, skin, liver, kidneys, hematopoietic, nervous and respiratory systems.[33]
Common controls used for hydrazine include process enclosure, local exhaust ventilation andpersonal protective equipment(PPE).[33]Guidelines for hydrazine PPE include non-permeable gloves and clothing, indirect-vent splash resistant goggles, face shield and in some cases a respirator.[40]The use of respirators for the handling of hydrazine should be the last resort as a method of controlling worker exposure. In cases where respirators are needed, proper respirator selection and a complete respiratory protection program consistent withOSHAguidelines should be implemented.[33]
ForUSAFpersonnel, Air Force Occupational Safety and Health (AFOSH) Standard 48-8, Attachment 8 reviews the considerations for occupational exposure to hydrazine in missile, aircraft and spacecraft systems. Specific guidance for exposure response includes mandatory emergency shower and eyewash stations and a process for decontaminating protective clothing. The guidance also assigns responsibilities and requirements for proper PPE, employee training, medical surveillance and emergency response.[41]USAF bases requiring the use of hydrazine generally have specific base regulations governing local requirements for safe hydrazine use and emergency response.
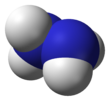
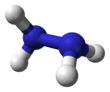
Molecular structure[edit]
Hydrazine,H2N−NH2,contains two amine groupsNH2connected by a single bond between the two nitrogen atoms. EachN−NH2subunit is pyramidal. The structure of the free molecules was determined bygas electron diffractionandmicrowave spectroscopy.The N–N single bond length is 1.447(2)Å(144.7(2)pm), the N-H distance is 1.015(2)Å,the N-N-H angles are 106(2)° and 112(2)°, the H-N-H angle is 107°.[42]The molecule adopts agauche conformationwith a torsion angle of 91(2)° (dihedral angle between the planes containing the N-N bond and the bisectors of the H-N-H angles). Therotational barrieris twice that ofethane.These structural properties resemble those of gaseoushydrogen peroxide,which adopts a "skewed"anticlinalconformation, and also experiences a strong rotational barrier.
The structure of solid hydrazine was determined by X-ray diffraction. In this phase the N-N bond has a length of 1.46Åand the nearest non-bonded distances are 3.19, 3.25 and 3.30Å.[43]
Synthesis and production[edit]
Diverse synthetic pathways for hydrazine production have been developed.[9]The key step is the creation of theN–N single bond. The many routes can be divided into those that use chlorine oxidants (and generate salt) and those that do not.
Oxidation of ammonia via oxaziridines from peroxide[edit]
Hydrazine can be synthesized from ammonia and hydrogen peroxide with a ketone catalyst, in a procedure called thePeroxide process(sometimes called Pechiney-Ugine-Kuhlmann process, the Atofina–PCUK cycle, or ketazine process).[9]The net reaction is:[44]
- 2 NH3+ H2O2→ N2H4+ 2 H2O
In this route, the ketone and ammonia first condense to give theimine,which is oxidised by hydrogen peroxide to theoxaziridine,a three-membered ring containing carbon, oxygen, and nitrogen. Next, the oxaziridine gives thehydrazonebytreatment with ammonia,which process creates the nitrogen-nitrogen single bond. This hydrazone condenses with one more equivalent of ketone.
The resultingazineis hydrolyzed to give hydrazine and regenerate the ketone,methyl ethyl ketone:
Unlike most other processes, this approach does not produce a salt as a by-product.[45]
Chlorine-based oxidations[edit]
TheOlin Raschig process,first announced in 1907, produces hydrazine fromsodium hypochlorite(the active ingredient in manybleaches) and ammonia without the use of a ketone catalyst. This method relies on the reaction ofmonochloraminewithammoniato create theN–Nsingle bondas well as ahydrogen chloridebyproduct:[12]
- NH2Cl + NH3→ N2H4+ HCl
Related to the Raschig process,ureacan be oxidized instead of ammonia. Again sodium hypochlorite serves as the oxidant. The net reaction is shown:[46]
- (NH2)2CO + NaOCl + 2 NaOH → N2H4+ H2O + NaCl + Na2CO3
The process generates significant by-products and is mainly practised in Asia.[9]
TheBayer Ketazine Processis the predecessor to the peroxide process. It employs sodium hypochlorite as oxidant instead of hydrogen peroxide. Like all hypochlorite-based routes, this method produces an equivalent of salt for each equivalent of hydrazine.[9]
Reactions[edit]
Acid-base behavior[edit]

Hydrazine forms amonohydrateN2H4·H2Othat is denser (1.032 g/cm3) than theanhydrousformN2H4(1.021 g/cm3). Hydrazine hasbasic(alkali) chemical properties comparable to those ofammonia:[47]
- N2H4+ H2O → [N2H5]++ OH−,Kb= 1.3 × 10−6,pKb= 5.9
(for ammoniaKb= 1.78 × 10−5)
It is difficult to diprotonate:[48]
- [N2H5]++ H2O → [N2H6]2++ OH−,Kb= 8.4 × 10−16,pKb= 15
Exposure to extremely strong bases or alkali metals generates deprotonated hydrazide salts. Most explode on exposure to air or moisture.[49]
Redox reactions[edit]
Ideally, the combustion of hydrazine in oxygen produces nitrogen and water:
- N2H4+ O2→ N2+ 2 H2O
An excess of oxygen gives oxides of nitrogen, includingnitrogen monoxideandnitrogen dioxide:
- N2H4+ 2 O2→ 2 NO + 2 H2O
- N2H4+ 3 O2→ 2 NO2+ 2 H2O
The heat of combustion of hydrazine in oxygen (air) is 19.41 MJ/kg (8345 BTU/lb).[50]
Hydrazine is a convenient reductant because the by-products are typically nitrogen gas and water. This property makes it useful as anantioxidant,an oxygenscavenger,and acorrosion inhibitorin water boilers and heating systems. It also directly reduces salts of less active metals (e.g., bismuth, arsenic, copper, mercury, silver, lead, platinum, and palladium) to the element.[51]That property has commercial application inelectrolessnickelplating andplutoniumextraction fromnuclear reactor waste.Some colour photographic processes also use a weak solution of hydrazine as a stabilising wash, as it scavengesdye couplerand unreacted silver halides. Hydrazine is the most common and effective reducing agent used to convertgraphene oxide(GO) to reduced graphene oxide (rGO) via hydrothermal treatment.[52]
Hydrazinium salts[edit]
Hydrazine can beprotonatedto form various solid salts of thehydraziniumcation[N2H5]+,by treatment with mineral acids. A common salt ishydrazinium hydrogensulfate,[N2H5]+[HSO4]−.[53]Hydrazinium hydrogensulfate was investigated as a treatment of cancer-inducedcachexia,but proved ineffective.[54]
Double protonation gives the hydraziniumdicationor hydrazinediium,[N2H6]2+,of which various salts are known.[55]
Organic chemistry[edit]
Hydrazines are part of manyorganic syntheses,often those of practical significance inpharmaceuticals(see applications section), as well as in textiledyesand in photography.[9]
Hydrazine is used in theWolff–Kishner reduction,a reaction that transforms thecarbonylgroup of aketoneinto amethylene bridge(or analdehydeinto amethyl group) via ahydrazoneintermediate. The production of the highly stabledinitrogenfrom the hydrazine derivative helps to drive the reaction.
Being bifunctional, with two amines, hydrazine is a key building block for the preparation of many heterocyclic compounds via condensation with a range of difunctionalelectrophiles.With2,4-pentanedione,it condenses to give the3,5-dimethylpyrazole.[56]In theEinhorn-Brunner reactionhydrazines react with imides to givetriazoles.
Being a good nucleophile,N2H4can attack sulfonyl halides and acyl halides.[57]Thetosylhydrazinealso forms hydrazones upon treatment with carbonyls.
Hydrazine is used to cleaveN-alkylated phthalimide derivatives. This scission reaction allows phthalimide anion to be used as amine precursor in theGabriel synthesis.[58]
Hydrazone formation[edit]
Illustrative of the condensation of hydrazine with a simple carbonyl is its reaction with acetone to give theacetone azine.The latter reacts further with hydrazine to yieldacetone hydrazone:[59]
- 2 (CH3)2CO + N2H4→ 2 H2O + ((CH3)2C=N)2
- ((CH3)2C=N)2+ N2H4→ 2 (CH3)2C=NNH2
The propanone azine is an intermediate in the Atofina-PCUK process.Directalkylationof hydrazines withalkyl halidesin the presence of base yields alkyl-substituted hydrazines, but the reaction is typically inefficient due to poor control on level of substitution (same as in ordinaryamines). The reduction ofhydrazonesto hydrazines present a clean way to produce 1,1-dialkylated hydrazines.
In a related reaction, 2-cyanopyridines react with hydrazine to form amide hydrazides, which can be converted using1,2-diketonesintotriazines.
Biochemistry[edit]
Hydrazine is the intermediate in the anaerobic oxidation of ammonia (anammox) process.[60]It is produced by some yeasts and the open ocean bacterium anammox (Brocadia anammoxidans).[61]
Thefalse morelproduces the poisongyromitrinwhich is an organic derivative of hydrazine that is converted tomonomethylhydrazineby metabolic processes. Even the most popular edible "button" mushroomAgaricus bisporusproduces organic hydrazine derivatives, includingagaritine,ahydrazine derivativeof an amino acid, andgyromitrin.[62][63]
History[edit]
The name "hydrazine" was coined byEmil Fischerin 1875; he was trying to produce organic compounds that consisted of mono-substituted hydrazine.[64]By 1887,Theodor Curtiushad produced hydrazine sulfate by treating organic diazides with dilute sulfuric acid; however, he was unable to obtain pure hydrazine, despite repeated efforts.[65][66][67]Pure anhydrous hydrazine was first prepared by the Dutch chemistLobry de Bruynin 1895.[68][69][70]
See also[edit]
- Nitrous oxide fuel blend– Class of liquid rocket propellants
- USA-193– U.S. military satellite (2006–2008)
References[edit]
- ^"NIOSH Guide—Hydrazine".Centers for Disease Control.Retrieved16 Aug2012.
- ^ab"hydrazine—PubChem Public Chemical Database".The PubChem Project.USA: National Center for Biotechnology Information.
- ^abcdefNIOSH Pocket Guide to Chemical Hazards."#0329".National Institute for Occupational Safety and Health(NIOSH).
- ^Hall HK, et al. (1957). "Correlation of the Base Strengths of Amines1".J. Am. Chem. Soc.79(20): 5441.doi:10.1021/ja01577a030.
- ^Greenwood NN,Earnshaw A (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- ^Martel B, Cassidy K, et al. (2004).Chemical Risk Analysis: A Practical Handbook.Amsterdam: Butterworth–Heinemann. p. 361.ISBN978-1-903996-65-2.OCLC939257974.
- ^"Hydrazine".Immediately Dangerous to Life or Health Concentrations (IDLH).National Institute for Occupational Safety and Health(NIOSH).
- ^Tsubakizaki S, Takada M, Gotou H, Mawatari K, Ishihara N, Kai R (2009)."Alternatives to Hydrazine in Water Treatment at Thermal Power Plants"(PDF).Mitsubishi Heavy Industries Technical Review.6(2): 43–47.
- ^abcdefghiSchirmann JP, Bourdauducq P (2001). "Hydrazine".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a13_177.ISBN3-527-30673-0.
- ^"A Summary of NASA and USAF Hypergolic Propellant Related Spills and Fires"(PDF).Kennedy Space Center.
- ^Suggs HJ, Luskus LJ, Kilian HJ, Mokry JW (1979)."Exhaust Gas Composition of the F-16 Emergency Power Unit"(technical report).USAF.SAM-TR-79-2. Archived fromthe originalon 4 March 2016.Retrieved23 Jan2019.
- ^abAdams R, Brown BK (1922). "Hydrazine Sulfate".Org. Synth.2:37.doi:10.15227/orgsyn.002.0037.S2CID221547391.
- ^Toki T, Koyanagi T, Yoshida K, Yamamoto K, Morita M (1994)."Hydrazine compounds useful as pesticides"(US patent). Ishihara Sangyo Kaisha Ltd (original assignee). US5304657A.
- ^ab"Liquid asset".The Engineer.Centaur Media plc. 15 Jan 2008.Retrieved23 Jan2019.
- ^abKnapton JD, Stobie IC, Elmore L (Mar 1993)."A Review of the Bulk-Loaded Liquid Propellant Gun Program for Possible Relevance to the Electrothermal Chemical Propulsion Program"(PDF).Army Research Laboratory.ADA263143.Archived(PDF)from the original on March 7, 2020.
- ^"Ground Servicing of Aircraft and Static Grounding/Bonding"(PDF).USAF(technical manual). 13 Mar 2017. TO 00-25-172.Retrieved23 Nov2018.
- ^abClark JD(23 May 2018).Ignition!: An Informal History of Liquid Rocket Propellants.Rutgers University Press. p. 302.ISBN978-0-8135-9918-2.
- ^T. W. Price, D. D. Evans.Technical Report 32-7227 The Status of Monopropellant Hydrazine Technology(PDF)(Report). National Aeronautics and Space Administration (NASA). p. 1.Retrieved22 February2022.
- ^"The Nedelin Catastrophe, Part 1".28 October 2014.Archivedfrom the original on 15 February 2022.Retrieved15 February2022.
- ^Haws JL, Harden DG (1965). "Thermodynamic Properties of Hydrazine".Journal of Spacecraft and Rockets.2(6): 972–974.Bibcode:1965JSpRo...2..972H.doi:10.2514/3.28327.
- ^Vieira R, Pham-Huu C, Kellera N, Ledouxa MJ (2002). "New carbon nanofiber/graphite felt composite for use as a catalyst support for hydrazine catalytic decomposition".Chem. Comm.44(9): 954–955.doi:10.1039/b202032g.PMID12123065.
- ^Chen X, Zhang T, Xia L, Li T, Zheng M, Wu Z, Wang X, Wei Z, Xin Q, Li C (Apr 2002). "Catalytic Decomposition of Hydrazine over Supported Molybdenum Nitride Catalysts in a Monopropellant Thruster".Catalysis Letters.79:21–25.doi:10.1023/A:1015343922044.S2CID92094908.
- ^"BIG-IP logout page".eso-io.Archived fromthe originalon June 23, 2008.RetrievedMay 20,2020.
- ^Valera-Medina A, Xiao H, Owen-Jones M, David WI, Bowen PJ (2018-11-01)."Ammonia for power".Progress in Energy and Combustion Science.69:63–102.doi:10.1016/j.pecs.2018.07.001.ISSN0360-1285.S2CID106214840.
- ^Mitchell MC, Rakoff RW, Jobe TO, Sanchez DL, Wilson B (2007). "Thermodynamic analysis of equations of state for the monopropellant hydrazine".Journal of Thermophysics and Heat Transfer.21(1): 243–246.doi:10.2514/1.22798.
- ^"Hydrazine ban could cost Europe's space industry billions".SpaceNews.2017-10-25.Retrieved2022-08-19.
- ^"International research projects | Ministry of Business, Innovation & Employment".mbie.govt.nz.Retrieved2022-08-19.
- ^Urban V (2022-07-15)."Dawn Aerospace granted €1.4 million by EU for green propulsion technology".SpaceWatch.Global.Retrieved2022-08-19.
- ^Berger E (2022-07-19)."Two companies join SpaceX in the race to Mars, with a launch possible in 2024".Ars Technica.Retrieved2022-08-19.
- ^"Launcher to develop orbital transfer vehicle".SpaceNews.2021-06-15.Retrieved2022-08-19.
- ^"Dawn Aerospace validates B20 Thrusters in space – Bits&Chips".Retrieved2022-08-19.
- ^"Dawn B20 Thrusters Proven In Space".Dawn Aerospace.Retrieved2022-08-19.
- ^abcdef"Occupational Safety and Health Guideline for Hydrazine—Potential Human Carcinogen"(PDF).NIOSH.1988.Retrieved23 Nov2018.
- ^ab"Hydrazine 302-01-2"(PDF).US EPA.Retrieved23 Nov2018.
- ^ab"International Programme on Chemical Safety—Health and Safety Guide No. 56—Hydrazine".IPCS INCHEM.Geneva:WHO.1991.Retrieved24 Nov2018.
- ^abcd"Occupational Chemical Database—Hydrazine".osha.gov.OSHA.Retrieved24 Nov2018.
- ^"Hydrazine"(PDF).IARC.Jun 2018. Archived fromthe original(PDF)on 26 November 2020.Retrieved23 Nov2018.
- ^Institute of Medicine (2005). "Ch. 9: Hydrazines and Nitric Acid".Gulf War and Health: Fuels, Combustion Products, and Propellants.Vol. 3. Washington, DC: The National Academies Press. p. 347.doi:10.17226/11180.ISBN978-0-309-09527-3.S2CID228274601.
- ^"Recommendation from the Scientific Committee on Occupational Exposure Limits for Hydrazine"(PDF).European Commission.Aug 2010.Retrieved23 Nov2018.
- ^ab"Hazardous Substance Fact Sheet—Hydrazine"(PDF).New Jersey Department of Public Health.Nov 2009.Retrieved23 Nov2018.
- ^ab"Air Force Occupational Safety and Health (AFOSH) Standard 48-8"(PDF).USAF.1 Sep 1997.Retrieved23 Nov2018.
- ^Kohata K, Fukuyama T, Kuchitsu K (March 1982). "Molecular structure of hydrazine as studied by gas electron diffraction".The Journal of Physical Chemistry.86(5): 602–606.doi:10.1021/j100394a005.ISSN0022-3654.
- ^Collin RL, Lipscomb WN (1951-01-01)."The crystal structure of hydrazine".Acta Crystallographica.4(1): 10–14.Bibcode:1951AcCry...4...10C.doi:10.1107/s0365110x51000027.ISSN0365-110X.
- ^Matar S, Hatch LF (2001).Chemistry of Petrochemical Processes(2nd ed.). Burlington: Gulf Professional Publishing. p. 148.ISBN978-1-4933-0346-5.OCLC990470096– via Elsevier.
- ^Riegel ER, Kent JA (2003)."Hydrazine".Riegel's handbook of industrial chemistry(10th ed.). New York: Springer Science & Business Media. p. 192.ISBN978-0-306-47411-8.OCLC55023601.
- ^"Hydrazine: Chemical product info".chemindustry.ru.Archived fromthe originalon 22 January 2018.Retrieved8 Jan2007.
- ^Handbook of Chemistry and Physics(83rd ed.). CRC Press. 2002.
- ^Holleman AF, Wiberg E, Wiberg N (2001).Inorganic chemistry(1st Eng. ed.). San Diego: Academic Press.ISBN978-0-12-352651-9.OCLC813400418.
- ^Eugene F. Rothgery (2004), "Hydrazine and its derivatives",Kirk-Othmer Encyclopedia of Chemical Technology,New York: John Wiley,doi:10.1002/0471238961.0825041819030809.a01.pub2,ISBN9780471238966
- ^"Hydrazine—Chemical Hazard Properties Table"(PDF).NOAA.gov.1999.
- ^Audrieth LF, Kleinberg J (1953).Non-aqueous solvents.New York: John Wiley & Sons. p. 133.LCCN52-12057.
- ^Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007). "Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide".Carbon.45(7): 1558–1565.Bibcode:2007Carbo..45.1558S.doi:10.1016/j.carbon.2007.02.034.S2CID14548921.
- ^"HYDRAZINE SULFATE".hazard.Retrieved22 Jan2019.
- ^Gagnon B, Bruera E (May 1998). "A review of the drug treatment of cachexia associated with cancer".Drugs.55(5): 675–88.doi:10.2165/00003495-199855050-00005.PMID9585863.S2CID22180434.
- ^"Diazanediium".CharChem.Retrieved22 Jan2019.
- ^Wiley RH, Hexner PE (1951). "3,5-Dimethylpyrazole".Org. Synth.31:43.doi:10.15227/orgsyn.031.0043.
- ^Friedman L, Litle RL, Reichle WR (1960). "p-Toluenesulfonyl Hydrazide".Org. Synth.40:93.doi:10.15227/orgsyn.040.0093.
- ^Weinshenker NM, Shen CM, Wong JY (1977). "Polymeric Carbodiimide. Preparation".Org. Synth.56:95.doi:10.15227/orgsyn.056.0095.
- ^Day AC, Whiting MC (1970). "Acetone Hydrazone".Organic Syntheses.50:3.doi:10.15227/orgsyn.050.0003.
- ^Strous M, Jetten MS (2004). "Anaerobic Oxidation of Methane and Ammonium".Annu Rev Microbiol.58:99–117.doi:10.1146/annurev.micro.58.030603.123605.hdl:2066/60186.PMID15487931.
- ^Handwerk B (9 Nov 2005)."Bacteria Eat Human Sewage, Produce Rocket Fuel".National Geographic.Retrieved12 Nov2007– via Wild Singapore.
- ^Hashida C, Hayashi K, Jie L, Haga S, Sakurai M, Shimizu H (1990). "[Quantities of agaritine in mushrooms (Agaricus bisporus) and the carcinogenicity of mushroom methanol extracts on the mouse bladder epithelium] ".Nippon Koshu Eisei Zasshi(in Japanese).37(6): 400–5.PMID2132000.
- ^Sieger AA, ed. (1 Jan 1998)."Spore Prints #338".Bulletin of the Puget Sound Mycological Society.Retrieved13 Oct2008.
- ^Fischer E (1875)."Über aromatische Hydrazinverbindungen"[On aromatic hydrazine compounds].Ber. Dtsch. Chem. Ges.8:589–594.doi:10.1002/cber.187500801178.
- ^Curtius T (1887)."Über das Diamid (Hydrazin)"[On diamide (hydrazine)].Ber. Dtsch. Chem. Ges.20:1632–1634.doi:10.1002/cber.188702001368.
- ^Curtius T, Jay R (1889)."Diazo- und Azoverbindungen der Fettreihe. IV. Abhandlung. über das Hydrazin"[Diazo- and azo- compounds of alkanes. Fourth treatise. On hydrazine]. In Erdmann OL (ed.).Journal für praktische Chemie.Vol. 147. Verlag von Johann Ambrosius Barth.On p. 129, Curtius admits: "Das freie DiamidNH2-NH2ist noch nicht analysiert worden."[Free hydrazine has not been analyzed yet.]
- ^Curtius T, Schulz H (1890)."Über Hydrazinehydrat und die Halogenverbindungen des Diammoniums"[On hydrazine hydrate and the halogen compounds of diammonium].Journal für praktische Chemie.Vol. 150. pp. 521–549.
- ^Lobry de Bruyn CA (1894). "Sur l'hydrazine (diamide) libre" [On free hydrazine (diamide)].Recl. Trav. Chim. Pays-Bas.13(8): 433–440.doi:10.1002/recl.18940130816.
- ^Lobry de Bruyn CA (1895). "Sur l'hydrate d'hydrazine" [On the hydrate of hydrazine].Recl. Trav. Chim. Pays-Bas.14(3): 85–88.doi:10.1002/recl.18950140302.
- ^Lobry de Bruyn CA (1896). "L'hydrazine libre I" [Free hydrazine, Part 1].Recl. Trav. Chim. Pays-Bas.15(6): 174–184.doi:10.1002/recl.18960150606.