Hydronium
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
oxonium
| |||
| Other names
hydronium ion
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 141 | |||
PubChemCID
|
|||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| H3O+ | |||
| Molar mass | 19.023g·mol−1 | ||
| Acidity(pKa) | 0 | ||
| Conjugate base | Water | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Inchemistry,hydronium(hydroxoniumin traditionalBritish English) is thecation[H3O]+,also written asH3O+,the type ofoxonium ionproduced byprotonationofwater.It is often viewed as the positive ion present when anArrhenius acidis dissolved in water, as Arrhenius acidmoleculesinsolutiongive up aproton(a positivehydrogenion,H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueousH+and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3,the Zundel cation, which is a symmetric dihydrate, H+(H2O)2,and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4.[1][2]Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form.[3][4][5][6][non-primary source needed]For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.[2]
Relation to pH[edit]
Themolar concentrationof hydronium orH+ions determines a solution'spHaccording to
- pH = -log([H3O+]/M)
where M = mol/L. The concentration ofhydroxideions analogously determines a solution'spOH.The molecules in pure waterauto-dissociateinto aqueous protons and hydroxide ions in the following equilibrium:
- H2O ⇌ OH−(aq) + H+(aq)
In pure water, there is an equal number of hydroxide andH+ions, so it is a neutral solution. At 25 °C (77 °F), pure water has a pH of 7 and a pOH of 7 (this varies when the temperature changes: seeself-ionization of water). A pH value less than 7 indicates an acidic solution, and a pH value more than 7 indicates a basic solution.[7]
Nomenclature[edit]
According toIUPAC nomenclature of organic chemistry,the hydronium ion should be referred to asoxonium.[8]Hydroxoniummay also be used unambiguously to identify it.[citation needed]
Anoxonium ionis any cation containing a trivalent oxygen atom.
Structure[edit]
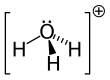
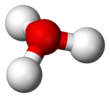
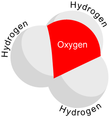
SinceO+and N have the same number of electrons,H3O+isisoelectronicwithammonia.As shown in the images above,H3O+has atrigonal pyramidal molecular geometrywith the oxygen atom at its apex. TheH−O−Hbond angleis approximately 113°,[9][10]and the center of mass is very close to the oxygen atom. Because the base of the pyramid is made up of three identical hydrogen atoms, theH3O+molecule'ssymmetric topconfiguration is such that it belongs to theC3vpoint group.Because of this symmetry and the fact that it has a dipole moment, the rotationalselection rulesare ΔJ= ±1 and ΔK= 0. Thetransition dipolelies along thec-axis and, because the negative charge is localized near the oxygen atom, the dipole moment points to the apex, perpendicular to the base plane.[citation needed]
Acids and acidity[edit]
The hydrated proton is very acidic: at 25 °C, itspKais approximately 0.[11]The values commonly given for pKaaq(H3O+) are 0 or –1.74. The former uses the convention that the activity of the solvent in a dilute solution (in this case, water) is 1, while the latter uses the value of the concentration of water in the pure liquid of 55.5 M. Silverstein has shown that the latter value is thermodynamically unsupportable.[12]The disagreement comes from the ambiguity that to define pKaof H3O+in water, H2O has to act simultaneously as a solute and the solvent. The IUPAC has not given an official definition of pKathat would resolve this ambiguity. Burgot has argued that H3O+(aq) + H2O (l) ⇄ H2O (aq) + H3O+(aq) is simply not a thermodynamically well-defined process. For an estimate of pKaaq(H3O+), Burgot suggests taking the measured value pKaEtOH(H3O+) = 0.3, the pKaof H3O+in ethanol, and applying the correlation equation pKaaq= pKaEtOH– 1.0 (± 0.3) to convert the ethanol pKato an aqueous value, to give a value of pKaaq(H3O+) = –0.7 (± 0.3).[13]On the other hand, Silverstein has shown that Ballinger and Long's experimental results[14]support a pKaof 0.0 for the aqueous proton.[15]Neils and Schaertel provide added arguments for a pKaof 0.0[16]
The aqueous proton isthe most acidic species that can exist in water(assuming sufficient water for dissolution): any stronger acid will ionize and yield a hydrated proton. The acidity ofH+(aq) is the implicit standard used to judge the strength of an acid in water:strong acidsmust be better proton donors thanH+(aq), as otherwise a significant portion of acid will exist in a non-ionized state (i.e.: a weak acid). UnlikeH+(aq) in neutral solutions that result from water's autodissociation, in acidic solutions,H+(aq) is long-lasting and concentrated, in proportion to the strength of the dissolved acid.[citation needed]
pH was originally conceived to be a measure of thehydrogen ionconcentration of aqueous solution.[17]Virtually all such free protons are quickly hydrated; acidity of an aqueous solution is therefore more accurately characterized by its concentration ofH+(aq). In organic syntheses, such as acid catalyzed reactions, the hydronium ion (H3O+) is used interchangeably with theH+ion; choosing one over the other has no significant effect on the mechanism of reaction.[citation needed]
Solvation[edit]
Researchers have yet to fully characterize thesolvationof hydronium ion in water, in part because many different meanings of solvation exist. Afreezing-point depressionstudy determined that the mean hydration ion in cold water is approximatelyH3O+(H2O)6:[18]on average, each hydronium ion is solvated by 6 water molecules which are unable to solvate other solute molecules.[citation needed]
Some hydration structures are quite large: theH3O+(H2O)20magic ion number structure (calledmagic numberbecause of its increased stability with respect to hydration structures involving a comparable number of water molecules – this is a similar usage of the termmagic numberas innuclear physics) might place the hydronium inside adodecahedralcage.[19]However, more recentab initio methodmolecular dynamics simulations have shown that, on average, the hydrated proton resides on the surface of theH3O+(H2O)20cluster.[20]Further, several disparate features of these simulations agree with their experimental counterparts suggesting an alternative interpretation of the experimental results.[citation needed]
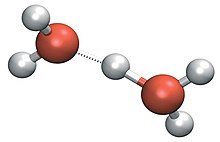
Two other well-known structures are theZundel cationand theEigen cation.The Eigen solvation structure has the hydronium ion at the center of anH9O+4complex in which the hydronium is stronglyhydrogen-bondedto three neighbouring water molecules.[21]In the ZundelH5O+2complex the proton is shared equally by two water molecules in asymmetric hydrogen bond.[22]Recent work indicates that both of these complexes represent ideal structures in a more general hydrogen bond network defect.[23]
Isolation of the hydronium ion monomer in liquid phase was achieved in a nonaqueous, low nucleophilicitysuperacidsolution (HF−SbF5SO2). The ion was characterized by high resolution17Onuclear magnetic resonance.[24]
A 2007 calculation of theenthalpiesandfree energiesof the various hydrogen bonds around the hydronium cation in liquid protonated water[25]at room temperature and a study of theproton hoppingmechanism usingmolecular dynamicsshowed that the hydrogen-bonds around the hydronium ion (formed with the three waterligandsin the firstsolvation shellof the hydronium) are quite strong compared to those of bulk water.[citation needed]
A new model was proposed by Stoyanov based oninfrared spectroscopyin which the proton exists as anH13O+6ion. The positive charge is thus delocalized over 6 water molecules.[26]
Solid hydronium salts[edit]
For manystrong acids,it is possible to form crystals of their hydronium salt that are relatively stable. These salts are sometimes calledacid monohydrates.As a rule, any acid with anionization constantof 109or higher may do this. Acids whose ionization constants are below 109generally cannot form stableH3O+salts. For example,nitric acidhas an ionization constant of 101.4,and mixtures with water at all proportions are liquid at room temperature. However,perchloric acidhas an ionization constant of 1010,and if liquid anhydrous perchloric acid and water are combined in a 1:1 molar ratio, they react to form solidhydronium perchlorate(H3O+·ClO−4).[citation needed]
The hydronium ion also forms stable compounds with thecarborane superacidH(CB11H(CH3)5Br6).[27]X-ray crystallographyshows aC3vsymmetryfor the hydronium ion with each proton interacting with a bromine atom each from three carborane anions 320pmapart on average. The[H3O] [H(CB11HCl11)]salt is also soluble inbenzene.In crystals grown from a benzene solution the solvent co-crystallizes and aH3O·(C6H6)3cation is completely separated from the anion. In the cation three benzene molecules surround hydronium formingpi-cation interactionswith the hydrogen atoms. The closest (non-bonding) approach of the anion at chlorine to the cation at oxygen is 348 pm.
There are also many known examples of salts containing hydrated hydronium ions, such as theH5O+2ion inHCl·2H2O,theH7O+3andH9O+4ions both found inHBr·4H2O.[28]
Sulfuric acidis also known to form a hydronium saltH3O+HSO−4at temperatures below 8.49 °C (47.28 °F).[29]
Interstellar H3O+[edit]
Hydronium is an abundantmolecular ionin the interstellar medium and is found in diffuse[30]and dense[31]molecular clouds as well as the plasma tails of comets.[32]Interstellar sources of hydronium observations include the regions of Sagittarius B2, Orion OMC-1, Orion BN–IRc2, Orion KL, and the comet Hale–Bopp.
Interstellar hydronium is formed by a chain of reactions started by the ionization ofH2intoH+2by cosmic radiation.[33]H3O+can produce eitherOH−orH2Othroughdissociative recombinationreactions, which occur very quickly even at the low (≥10 K) temperatures of dense clouds.[34]This leads to hydronium playing a very important role in interstellar ion-neutral chemistry.[citation needed]
Astronomers are especially interested in determining the abundance of water in various interstellar climates due to its key role in the cooling of dense molecular gases through radiative processes.[35]However,H2Odoes not have many favorable transitions for ground-based observations.[36]Although observations of HDO (thedeuterated version of water[37]) could potentially be used for estimatingH2Oabundances, the ratio of HDO toH2Ois not known very accurately.[36]
Hydronium, on the other hand, has several transitions that make it a superior candidate for detection and identification in a variety of situations.[36]This information has been used in conjunction with laboratory measurements of the branching ratios of the variousH3O+dissociative recombination reactions[34]to provide what are believed to be relatively accurateOH−andH2Oabundances without requiring direct observation of these species.[38][39]
Interstellar chemistry[edit]
As mentioned previously,H3O+is found in both diffuse and dense molecular clouds. By applying the reaction rate constants (α,β,andγ) corresponding to all of the currently available characterized reactions involvingH3O+,it is possible to calculatek(T) for each of these reactions. By multiplying thesek(T) by the relative abundances of the products, the relative rates (in cm3/s) for each reaction at a given temperature can be determined. These relative rates can be made in absolute rates by multiplying them by the[H2]2.[40]By assumingT= 10 Kfor a dense cloud andT= 50 Kfor a diffuse cloud, the results indicate that most dominant formation and destruction mechanisms were the same for both cases. It should be mentioned that the relative abundances used in these calculations correspond to TMC-1, a dense molecular cloud, and that the calculated relative rates are therefore expected to be more accurate atT= 10 K.The three fastest formation and destruction mechanisms are listed in the table below, along with their relative rates. Note that the rates of these six reactions are such that they make up approximately 99% of hydronium ion's chemical interactions under these conditions.[32]All three destruction mechanisms in the table below are classified asdissociative recombinationreactions.[41]
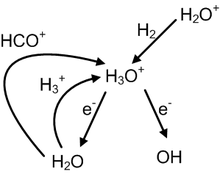
| Reaction | Type | Relative rate (cm3/s) | |
|---|---|---|---|
| at 10 K | at 50 K | ||
| H2+ H2O+→ H3O++ H | Formation | 2.97×10−22 | 2.97×10−22 |
| H2O + HCO+→ CO + H3O+ | Formation | 4.52×10−23 | 4.52×10−23 |
| H+3+ H2O → H3O++ H2 | Formation | 3.75×10−23 | 3.75×10−23 |
| H3O++ e−→ OH + H + H | Destruction | 2.27×10−22 | 1.02×10−22 |
| H3O++ e−→ H2O + H | Destruction | 9.52×10−23 | 4.26×10−23 |
| H3O++ e−→ OH + H2 | Destruction | 5.31×10−23 | 2.37×10−23 |
It is also worth noting that the relative rates for the formation reactions in the table above are the same for a given reaction at both temperatures. This is due to the reaction rate constants for these reactions havingβandγconstants of 0, resulting ink=αwhich is independent of temperature.[citation needed]
Since all three of these reactions produce eitherH2Oor OH, these results reinforce the strong connection between their relative abundances and that ofH3O+.The rates of these six reactions are such that they make up approximately 99% of hydronium ion's chemical interactions under these conditions.[citation needed]
Astronomical detections[edit]
As early as 1973 and before the first interstellar detection, chemical models of the interstellar medium (the first corresponding to a dense cloud) predicted that hydronium was an abundant molecular ion and that it played an important role in ion-neutral chemistry.[42]However, before an astronomical search could be underway there was still the matter of determining hydronium's spectroscopic features in the gas phase, which at this point were unknown. The first studies of these characteristics came in 1977,[43]which was followed by other, higher resolution spectroscopy experiments. Once several lines had been identified in the laboratory, the first interstellar detection of H3O+was made by two groups almost simultaneously in 1986.[31][36]The first, published in June 1986, reported observation of theJvt
K= 1−
1− 2+
1transition at307192.41 MHzin OMC-1 and Sgr B2. The second, published in August, reported observation of the same transition toward the Orion-KL nebula.[citation needed]
These first detections have been followed by observations of a number of additionalH3O+transitions. The first observations of each subsequent transition detection are given below in chronological order:
In 1991, the 3+
2− 2−
2transition at364797.427 MHzwas observed in OMC-1 and Sgr B2.[44]One year later, the 3+
0− 2−
0transition at396272.412 MHzwas observed in several regions, the clearest of which was the W3 IRS 5 cloud.[39]
The first far-IR 4−
3− 3+
3transition at 69.524 μm (4.3121 THz) was made in 1996 near Orion BN-IRc2.[45]In 2001, three additional transitions ofH3O+in were observed in the far infrared in Sgr B2; 2−
1− 1+
1transition at 100.577 μm (2.98073 THz), 1−
1− 1+
1at 181.054 μm (1.65582 THz) and 2−
0− 1+
0at 100.869 μm (2.9721 THz).[46]
See also[edit]
- Hydron(hydrogen cation)
- Hydride
- Hydrogen anion
- Hydrogen ion
- Grotthus mechanism
- Trifluorooxonium
- Law of dilution
References[edit]
- ^Reed, C.A. (2013)."Myths about the proton. The nature of H+ in condensed media".Acc. Chem. Res.46(11): 2567–2575.doi:10.1021/ar400064q.PMC3833890.PMID23875729.
- ^abSilverstein, Todd P. (2014). "The aqueous proton is hydrated by more than one water molecule: Is the hydronium ion a useful conceit?".J. Chem. Educ.91(4): 608–610.Bibcode:2014JChEd..91..608S.doi:10.1021/ed400559t.
- ^Thamer, M.; DeMarco, L.; Ramesha, K.; Mandel, A.; Tokmakoff, A. (2015)."Ultrafast 2D IR spectroscopy of the excess proton in liquid water".Science.350(6256): 78–82.Bibcode:2015Sci...350...78T.doi:10.1126/science.aab3908.PMID26430117.S2CID27074374.
- ^Daly Jr., C.A.; Streacker, L.M.; Sun, Y.; Pattenaude, S.R.; Hassanali, A.A.; Petersen, P.B.; et al. (2017). "Decomposition of the experimental Raman and IR spectra of acidic water into proton, special pair, and counterion contributions".J. Phys. Chem. Lett.8(21): 5246–5252.doi:10.1021/acs.jpclett.7b02435.PMID28976760.
- ^Dahms, F.; Fingerhut, B.P.; Nibbering, E.T.; Pines, E.; Elsaesser, T. (2017)."Large-amplitude transfer motion of hydrated excess protons mapped by ultrafast 2D IR spectroscopy".Science.357(6350): 491–495.Bibcode:2017Sci...357..491D.doi:10.1126/science.aan5144.PMID28705988.S2CID40492001.
- ^Fournier, J.A.; Carpenter, W.B.; Lewis, N.H.; Tokmakoff, A. (2018). "Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions".Nature Chemistry.10(9): 932–937.Bibcode:2018NatCh..10..932F.doi:10.1038/s41557-018-0091-y.OSTI1480907.PMID30061612.S2CID51882732.
- ^"pH and Water".United States Geological Survey.Retrieved9 November2021.
- ^"Table 17 Mononuclear parent onium ions".IUPAC.
- ^Tang, Jian; Oka, Takeshi (1999). "Infrared spectroscopy of H3O+:the v1fundamental band ".Journal of Molecular Spectroscopy.196(1): 120–130.Bibcode:1999JMoSp.196..120T.doi:10.1006/jmsp.1999.7844.PMID10361062.
- ^Bell, R. P. (1973).The Proton in Chemistry(2nd ed.). Ithaca: Cornell University Press. p. 15.
- ^Meister, Erich; Willeke, Martin; Angst, Werner; Togni, Antonio; Walde, Peter (2014). "Confusing Quantitative Descriptions of Brønsted-Lowry Acid-Base Equilibria in Chemistry Textbooks – A Critical Review and Clarifications for Chemical Educators".Helv. Chim. Acta.97(1): 1–31.doi:10.1002/hlca.201300321.
- ^Silverstein, T.P.; Heller, S.T. (2017). "pKa Values in the Undergraduate Curriculum: What Is the Real pKa of Water?".J. Chem. Educ.94(6): 690–695.Bibcode:2017JChEd..94..690S.doi:10.1021/acs.jchemed.6b00623.
- ^Burgot, Jean-Louis (1998)."PerspectiveNew point of view on the meaning and on the values of Ka○(H3O+,H2O) and Kb○(H2O, OH−) pairs in water ".The Analyst.123(2): 409–410.Bibcode:1998Ana...123..409B.doi:10.1039/a705491b.
- ^Ballinger, P.; Long, F.A. (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds".J. Am. Chem. Soc.82(4): 795–798.doi:10.1021/ja01489a008.
- ^Silverstein, T.P. (2014). "The aqueous proton is hydrated by more than one water molecule: Is the hydronium ion a useful conceit?".J. Chem. Educ.91(4): 608–610.Bibcode:2014JChEd..91..608S.doi:10.1021/ed400559t.
- ^"What is the pKa of Water".University of California, Davis.2015-08-09.
- ^Sorensen, S. P. L. (1909). "Ueber die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen".Biochemische Zeitschrift(in German).21:131–304.
- ^Zavitsas, A. A. (2001). "Properties of water solutions of electrolytes and nonelectrolytes".The Journal of Physical Chemistry B.105(32): 7805–7815.doi:10.1021/jp011053l.
- ^Hulthe, G.; Stenhagen, G.; Wennerström, O.; Ottosson, C-H. (1997). "Water cluster studied by electrospray mass spectrometry".Journal of Chromatography A.512:155–165.doi:10.1016/S0021-9673(97)00486-X.
- ^Iyengar, S. S.; Petersen, M. K.; Burnham, C. J.; Day, T. J. F.; Voth, G. A.; Voth, G. A. (2005)."The Properties of Ion-Water Clusters. I. The Protonated 21-Water Cluster"(PDF).The Journal of Chemical Physics.123(8): 084309.Bibcode:2005JChPh.123h4309I.doi:10.1063/1.2007628.PMID16164293.
- ^Zundel, G.; Metzger, H. (1968). "Energiebänder der tunnelnden Überschuß-Protonen in flüssigen Säuren. Eine IR-spektroskopische Untersuchung der Natur der Gruppierungen H502+".Zeitschrift für Physikalische Chemie.58(5_6): 225–245.doi:10.1524/zpch.1968.58.5_6.225.S2CID101048854.
- ^Wicke, E.; Eigen, M.; Ackermann, Th (1954). "Über den Zustand des Protons (Hydroniumions) in wäßriger Lösung".Zeitschrift für Physikalische Chemie.1(5_6): 340–364.doi:10.1524/zpch.1954.1.5_6.340.
- ^Marx, D.; Tuckerman, M. E.; Hutter, J.; Parrinello, M. (1999). "The nature of the hydrated excess proton in water".Nature.397(6720): 601–604.Bibcode:1999Natur.397..601M.doi:10.1038/17579.S2CID204991299.
- ^Mateescu, G. D.; Benedikt, G. M. (1979). "Water and related systems. 1. The hydronium ion (H3O+). Preparation and characterization by high resolution oxygen-17 nuclear magnetic resonance ".Journal of the American Chemical Society.101(14): 3959–3960.doi:10.1021/ja00508a040.
- ^Markovitch, O.; Agmon, N. (2007)."Structure and Energetics of the Hydronium Hydration Shells"(PDF).The Journal of Physical Chemistry A.111(12): 2253–6.Bibcode:2007JPCA..111.2253M.CiteSeerX10.1.1.76.9448.doi:10.1021/jp068960g.PMID17388314.Archived fromthe original(PDF)on 2018-08-31.Retrieved2018-08-30.
- ^Stoyanov, Evgenii S.; Stoyanova, Irina V.; Reed, Christopher A. (January 15, 2010)."The Structure of the Hydrogen Ion (H+
aq) in Water ".Journal of the American Chemical Society.132(5): 1484–1485.doi:10.1021/ja9101826.PMC2946644.PMID20078058. - ^ Stoyanov, Evgenii S.; Kim, Kee-Chan; Reed, Christopher A. (2006)."The Nature of the H3O+Hydronium Ion in Benzene and Chlorinated Hydrocarbon Solvents. Conditions of Existence and Reinterpretation of Infrared Data ".Journal of the American Chemical Society.128(6): 1948–58.doi:10.1021/ja0551335.PMID16464096.S2CID33834275.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- ^I. Taesler and I. Olavsson (1968). "Hydrogen bond studies. XXI. The crystal structure of sulfuric acid monohydrate." Acta Crystallogr. B24, 299-304.https://doi.org/10.1107/S056774086800227X
- ^Faure, A.; Tennyson, J. (2003)."Rate coefficients for electron-impact rotational excitation of H3+and H3O+".Monthly Notices of the Royal Astronomical Society.340(2): 468–472.Bibcode:2003MNRAS.340..468F.doi:10.1046/j.1365-8711.2003.06306.x.
- ^abHollis, J. M.; Churchwell, E. B.; Herbst, E.; De Lucia, F. C. (1986). "An interstellar line coincident with the P(2,l) transition of hydronium (H3O+) ".Nature.322(6079): 524–526.Bibcode:1986Natur.322..524H.doi:10.1038/322524a0.S2CID4346975.
- ^abRauer, H (1997). "Ion composition and solar wind interaction: Observations of comet C/1995 O1 (Hale-Bopp)".Earth, Moon, and Planets.79:161–178.Bibcode:1997EM&P...79..161R.doi:10.1023/A:1006285300913.S2CID119953549.
- ^Vejby-Christensen, L.; Andersen, L. H.; Heber, O.; Kella, D.; Pedersen, H. B.; Schmidt, H. T.; Zajfman, D. (1997)."Complete Branching Ratios for the Dissociative Recombination of H2O+,H3O+,and CH3+".The Astrophysical Journal.483(1): 531–540.Bibcode:1997ApJ...483..531V.doi:10.1086/304242.
- ^abNeau, A.; Al Khalili, A.; Rosén, S.; Le Padellec, A.; Derkatch, A. M.; Shi, W.; Vikor, L.; Larsson, M.; Semaniak, J.; Thomas, R.; Någård, M. B.; Andersson, K.; Danared, H.; Af Ugglas, M. (2000). "Dissociative recombination of D3O+and H3O+:Absolute cross sections and branching ratios ".The Journal of Chemical Physics.113(5): 1762.Bibcode:2000JChPh.113.1762N.doi:10.1063/1.481979.
- ^Neufeld, D. A.; Lepp, S.; Melnick, G. J. (1995). "Thermal Balance in Dense Molecular Clouds: Radiative Cooling Rates and Emission-Line Luminosities".The Astrophysical Journal Supplement Series.100:132.Bibcode:1995ApJS..100..132N.doi:10.1086/192211.
- ^abcdWootten, A.; Boulanger, F.; Bogey, M.; Combes, F.; Encrenaz, P. J.; Gerin, M.;Ziurys, L.(1986). "A search for interstellar H3O+".Astronomy and Astrophysics.166:L15–8.Bibcode:1986A&A...166L..15W.PMID11542067.
- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "heavy water".doi:10.1351/goldbook.H02758
- ^Herbst, E.; Green, S.; Thaddeus, P.; Klemperer, W. (1977). "Indirect observation of unobservable interstellar molecules".The Astrophysical Journal.215:503–510.Bibcode:1977ApJ...215..503H.doi:10.1086/155381.hdl:2060/19770013020.S2CID121202097.
- ^abPhillips, T. G.; Van Dishoeck, E. F.; Keene, J. (1992)."Interstellar H3O+and its Relation to the O2and H2O- Abundances "(PDF).The Astrophysical Journal.399:533.Bibcode:1992ApJ...399..533P.doi:10.1086/171945.hdl:1887/2260.
- ^"H3O+formation reactions ".The UMIST Database for Astrochemistry.
- ^"Dissociative recombination | physics".Encyclopedia Britannica.Retrieved2021-09-30.
- ^Herbst, E.; Klemperer, W. (1973)."The formation and depletion of molecules in dense interstellar clouds".The Astrophysical Journal.185:505.Bibcode:1973ApJ...185..505H.doi:10.1086/152436.
- ^Schwarz, H.A. (1977). "Gas phase infrared spectra of oxonium hydrate ions from 2 to 5 μm".Journal of Chemical Physics.67(12): 5525.Bibcode:1977JChPh..67.5525S.doi:10.1063/1.434748.
- ^Wootten, A.; Turner, B. E.; Mangum, J. G.; Bogey, M.; Boulanger, F.; Combes, F.; Encrenaz, P. J.; Gerin, M. (1991). "Detection of interstellar H3O+– A confirming line ".The Astrophysical Journal.380:L79.Bibcode:1991ApJ...380L..79W.doi:10.1086/186178.
- ^Timmermann, R.; Nikola, T.; Poglitsch, A.; Geis, N.; Stacey, G. J.; Townes, C. H. (1996)."Possible discovery of the 70 μm {H3O+} 4−
3− 3+
3transition in Orion BN-IRc2 ".The Astrophysical Journal.463(2): L109.Bibcode:1996ApJ...463L.109T.doi:10.1086/310055. - ^Goicoechea, J. R.; Cernicharo, J. (2001)."Far-infrared detection of H3O+in Sagittarius B2 ".The Astrophysical Journal.554(2): L213.Bibcode:2001ApJ...554L.213G.doi:10.1086/321712.hdl:10261/192309.