Tonicity
This articleneeds additional citations forverification.(February 2018) |

Inchemical biology,tonicityis a measure of the effectiveosmotic pressuregradient; thewater potentialof twosolutionsseparated by apartially-permeablecellmembrane.Tonicity depends on the relativeconcentrationof selective membrane-impermeablesolutesacross a cell membrane which determine the direction and extent of osmoticflux.It is commonly used when describing the swelling-versus-shrinking response ofcellsimmersed in an external solution.
Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affectingimbibition.
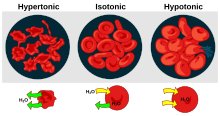
There are three classifications of tonicity that one solution can have relative to another:hypertonic,hypotonic,andisotonic.[1]A hypotonic solution example is distilled water.
Hypertonic solution
[edit]
A hypertonic solution has a greater concentration of non-permeatingsolutesthan another solution.[2]In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of acell membrane;a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than thecytosolinside the cell. When a cell is immersed in a hypertonic solution, osmotic pressure tends to force water to flow out of the cell in order to balance the concentrations of the solutes on either side of the cell membrane. The cytosol is conversely categorized as hypotonic, opposite of the outer solution.[3][4]
When plant cells are in a hypertonic solution, the flexible cell membrane pulls away from the rigidcell wall,but remains joined to the cell wall at points calledplasmodesmata.The cells often take on the appearance of apincushion,and the plasmodesmata almost cease to function because they become constricted, a condition known asplasmolysis.In plant cells the terms isotonic, hypotonic and hypertonic cannot strictly be used accurately because the pressure exerted by the cell wall significantly affects the osmotic equilibrium point.[5]
Some organisms have evolved intricate methods of circumventing hypertonicity. For example,saltwateris hypertonic to thefishthat live in it. Because the fish need a large surface area in theirgillsin contact with seawater forgas exchange,they lose water osmotically to the sea from gill cells. They respond to the loss by drinking large amounts of saltwater, and activelyexcretingthe excess salt.[6]This process is calledosmoregulation.[7]
Hypotonic solution
[edit]
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to thecytosol.Due toosmotic pressure,water diffuses into the cell, and the cell often appearsturgid,or bloated. For cells without acell wallsuch as animal cells, if the gradient is large enough, the uptake of excess water can produce enough pressure to inducecytolysis,orrupturingof the cell. When plant cells are in a hypotonic solution, the centralvacuoletakes on extra water and pushes the cell membrane against the cell wall. Due to the rigidity of the cell wall, it pushes back, preventing the cell from bursting. This is calledturgor pressure.[8]
Isotonicity
[edit]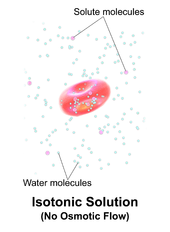
A solution is isotonic when its effectiveosmoleconcentration is the same as that of another solution. In biology, the solutions on either side of a cell membrane are isotonic if the concentration of solutes outside the cell is equal to the concentration of solutes inside the cell. In this case the cell neither swells nor shrinks because there is no concentration gradient to induce the diffusion of large amounts of water across the cell membrane. Water molecules freely diffuse through the plasma membrane in both directions, and as the rate of water diffusion is the same in each direction, the cell will neither gain nor lose water.
An iso-osmolar solution can be hypotonic if the solute is able to penetrate the cell membrane. For example, an iso-osmolarureasolution is hypotonic to red blood cells, causing theirlysis.This is due to urea entering the cell down its concentration gradient, followed by water. The osmolarity ofnormal saline,9 gramsNaCldissolved in water to a total volume of one liter, is a close approximation to the osmolarity of NaCl in blood (about 290mOsm/L). Thus, normal saline is almost isotonic to blood plasma. Neither sodium nor chloride ions can freely pass through the plasma membrane, unlikeurea.
See also
[edit]References
[edit]- ^Sperelakis, Nicholas (2011).Cell Physiology Source Book: Essentials of Membrane Biophysics.Academic Press. p. 288.ISBN978-0-12-387738-3.
- ^Buckley, Gabe (20 January 2017). "Hypertonic Solution". In Biologydictionary.net (ed.).Biology Dictionary(Online ed.). Biologydictionary.net.Retrieved19 August2021.
- ^LibreTexts Project: Medicine (18 July 2018). "3.3C - Tonicity".Anatomy and Physiology (Boundless)(Online ed.). med.libretexts.org/.Retrieved19 August2021.
- ^Argyropoulos, Christos; Rondon-Berrios, Helbert; Raj, Dominic S; Malhotra, Deepak; Agaba, Emmanuel I; Rohrscheib, Mark; Khitan, Zeid; Murata, Glen H; Shapiro, Joseph I.; Tzamaloukas, Antonios H (2 May 2016)."Hypertonicity: Pathophysiologic Concept and Experimental Studies".Cureus.8(5): e596.doi:10.7759/cureus.596.PMC4895078.PMID27382523.
- ^Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000). "Osmosis, Water Channels, and the Regulation of Cell Volume".Molecular Cell Biology(4th ed.). New York: W. H. Freeman and Company.Retrieved19 August2021.
- ^Soult, Allison (2020). "8.4 - Osmosis and Diffusion". In University of Kentucky (ed.).Chemistry for Allied Health.Open Education Resource (OER) LibreTexts Project.Retrieved19 August2021.
- ^Ortiz, RM (June 2001)."Osmoregulation in marine mammals".The Journal of Experimental Biology.204(Pt 11): 1831–44.doi:10.1242/jeb.204.11.1831.PMID11441026.
- ^"Definition — hypotonic".The Free Dictionary.Retrieved23 August2012.
