Iron-56
| General | |
|---|---|
| Symbol | 56Fe |
| Names | iron-56, 56Fe, Fe-56 |
| Protons(Z) | 26 |
| Neutrons(N) | 30 |
| Nuclide data | |
| Natural abundance | 91.754% |
| Isotope mass | 55.9349375(7)Da |
| Spin | 0+ |
| Excess energy | −60601.003±1.354keV |
| Binding energy | 492253.892±1.356keV |
| Isotopes of iron Complete table of nuclides | |
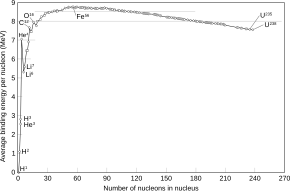
Iron-56(56Fe) is the most commonisotopeofiron.About 91.754% of all iron is iron-56.
Of allnuclides,iron-56 has the lowest mass pernucleon.With 8.8MeVbinding energyper nucleon, iron-56 is one of the most tightly bound nuclei.[1]
The high nuclear binding energy for56Fe represents the point where further nuclear reactions become energetically unfavorable. Because of this, it is among the heaviest elements formed instellar nucleosynthesisreactions in massive stars. These reactions fuse lighter elements like magnesium, silicon, and sulfur to form heavier elements. Among the heavier elements formed is56Ni,which subsequently decays to56Coand then56Fe.
Relationship to nickel-62
[edit]Nickel-62,a relatively rare isotope of nickel, has a highernuclear binding energyper nucleon; this is consistent with having a higher mass-per-nucleon because nickel-62 has a greater proportion ofneutrons,which are slightly more massive thanprotons.(See thenickel-62article for more). Light elements undergoingnuclear fusionand heavy elements undergoingnuclear fissionrelease energy as their nucleons bind more tightly, so62Ni might be expected to be common. However, duringstellar nucleosynthesisthe competition betweenphotodisintegrationandAlpha capturingcauses more56Nito be produced than62Ni (56Fe is produced later in the star's ejection shell as56Ni decays).
Although nickel-62 has a higher binding energy per nucleon, the conversion of 28 atoms of nickel-62 into 31 atoms of iron-56 releases0.011Daof energy. As theuniverseages, matter will slowly convert to ever more tightly bound nuclei, approaching56Fe, ultimately leading to the formation ofiron starsover ≈ 101500years, assuming anexpanding universe without proton decay.[2]
See also
[edit]References
[edit]- ^Nuclear Binding Energy
- ^Dyson, Freeman J. (1979). "Time without end: Physics and biology in an open universe".Reviews of Modern Physics.51(3): 447–460.Bibcode:1979RvMP...51..447D.doi:10.1103/RevModPhys.51.447.
- de Laeter, John Robert;Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003)."Atomic weights of the elements. Review 2000 (IUPAC Technical Report)".Pure and Applied Chemistry.75(6): 683–800.doi:10.1351/pac200375060683.
